
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 7PP
PRACTICE PROBLEM 6.7
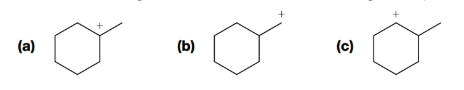
Rank the following carbocations in order of increasing stability:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
8
00
6
= 10
10
Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11.
If
you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than
unstable, you can pick any of them to redraw.)
Check
OH
stable
HO
stable
Ounstable
unstable
O
OH
stable
unstable
OH
80
F6
F5
stable
Ounstable
X
Save For Later
Sub
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C
ཀྭ་
A
F7
매
F8
F9
4
F10
Just try completing it and it should be straightforward according to the professor and TAs.
The grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.
Chapter 6 Solutions
Organic Chemistry
Ch. 6 - Prob. 1PPCh. 6 - Prob. 2PPCh. 6 - Prob. 3PPCh. 6 - Prob. 4PPCh. 6 - Prob. 5PPCh. 6 - PRACTICE PROBLEM 6.6
reactions that involve...Ch. 6 - PRACTICE PROBLEM 6.7 Rank the following...Ch. 6 - Prob. 8PPCh. 6 - Prob. 9PPCh. 6 - Prob. 10PP
Ch. 6 - Prob. 11PPCh. 6 - Prob. 12PPCh. 6 - Prob. 13PPCh. 6 - Prob. 14PPCh. 6 - Prob. 15PPCh. 6 - Prob. 16PPCh. 6 - Prob. 17PPCh. 6 - Prob. 18PPCh. 6 - Prob. 19PPCh. 6 - Prob. 20PCh. 6 - Prob. 21PCh. 6 - Prob. 22PCh. 6 - Prob. 23PCh. 6 - Prob. 24PCh. 6 - Listed below are several hypothetical nucleophilic...Ch. 6 - Prob. 26PCh. 6 - 6.27 Write conformational structures for the...Ch. 6 - 6.28 Consider the reaction of with .
(a) Would...Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - 1-Bromobicyclo[2, 2,1] heptane is extremely...Ch. 6 - When ethyl bromide reacts with potassium cyanide...Ch. 6 - Prob. 35PCh. 6 - Prob. 36PCh. 6 - When the alkyl bromides (listed here) were...Ch. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - 6.42 The reaction of chloroethane with water in...Ch. 6 - Prob. 43PCh. 6 - Prob. 44PCh. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - 1-Bromo[2.2.1] bicycloheptane is unreactive toward...Ch. 6 - Open the computer molecular model tided...Ch. 6 - Prob. 49PCh. 6 - Consider the solvolysis reaction of (1S,...Ch. 6 - 2. Consider the following sequence of reactions,...
Additional Science Textbook Solutions
Find more solutions based on key concepts
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
Approximately how many feet is the Missouri River above sea level? Height above sea level: _________ feet
Applications and Investigations in Earth Science (9th Edition)
Difference between alpha decay and beta decay must be explained. Concept introduction: In alpha decay, one of t...
Living By Chemistry: First Edition Textbook
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forward
- Predict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY