
Concept explainers
Interpretation:
The IUPAC names of the given compounds are to be determined.
Concept introduction:
The IUPAC name of
The longest carbon chain is numbered in a way so as to give a low numbering to the carbon with the halogen atom.
The substituents on the parent alkane are written alphabetically before the name of the parent alkane.
In cyclic compounds, the prefix ‘cyclo’ is used before the name of an alkane.
Substituents present on the same side of a ring are named with the prefix cis while those on opposite sides are named with the prefix‘trans’.
Answer to Problem 1PP
Solution:
Explanation of Solution
a)
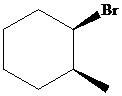
The parent alkane here is cyclohexane. The substituents are a bromine atom and a methyl group. Number the cyclohexane ring starting from the carbonwithbromine atom.
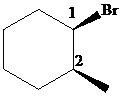
Putting the names of the substituents as the prefix alphabetically along with their positions before the name of the parent alkane, the IUPAC name can be written as
The two substituents are on the same side. Hence, the complete IUPAC name of the compound is
b)

The parent alkane here is cyclohexane. The substituents are a bromine atom and a methyl group. Number the cyclohexane ring starting from the carbon with the bromine atom. Both the bromine group and the methyl group are in equatorial position.
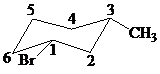
Putting the names of substituents as the prefix alphabetically along with their positions before the name of the parent alkane, the name can be written as
The two substituents are equatorial at the
Hence, the complete IUPAC name of the compound is
c)
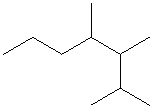
Select the longest continuous carbon chain and number it from that end, giving the lower number to the substituents.
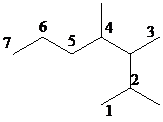
The parent chain has seven carbon atoms. Thus, the parent alkane is heptane. There are three methyl substituents at
Hence, the IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





