
LCPO CHEMISTRY W/MODIFIED MASTERING
8th Edition
ISBN: 9780135214756
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.26CP
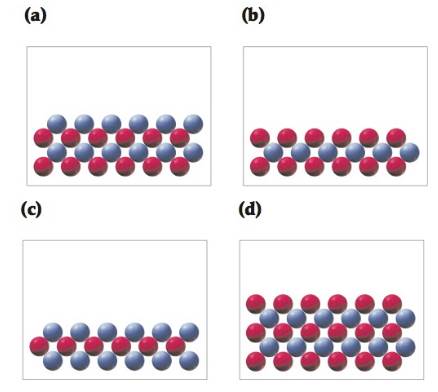
In the following drawings, red spheres represent cations and blue spheres represent anions. Match each of the drawings (a)—(d) with the following ionic compounds:
(i) Ca3(PO4)2
(ii) Li2CO3
(iii) FeCl2
(iv) MgSO4
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 6 Solutions
LCPO CHEMISTRY W/MODIFIED MASTERING
Ch. 6 - Prob. 6.1PCh. 6 - APPLY 6.2 Which of the following sets of ions are...Ch. 6 - Which atom or ion has the largest radius:...Ch. 6 - Conceptual APPLY 6.4 Which of the following...Ch. 6 - Use the periodic table to order the elements from...Ch. 6 - Given the orbital filling diagrams for the valence...Ch. 6 - Which has the largest third ionization energy: Be,...Ch. 6 - Conceptual APPLY 6.8 The figure on the right...Ch. 6 - Order the following elements from least to most...Ch. 6 - Conceptual APPLY 6.10 Which of the indicated three...
Ch. 6 - What electron configuration does the strontium...Ch. 6 - Prob. 6.12ACh. 6 - Prob. 6.13PCh. 6 - APPLY 6.14 Calculate the energy of electrostatic...Ch. 6 - Which substance has the largest lattice energy:...Ch. 6 - One of the following pictures represents NaCl and...Ch. 6 - Prob. 6.17PCh. 6 - What structural features do ionic liquids havethat...Ch. 6 - PROBLEM 6.18 Compare the following two ionic...Ch. 6 - PROBLEM 6.19 An ionic liquid consisting of a bulky...Ch. 6 - Where on the periodic table would you find the...Ch. 6 - Which of the following spheres is likely to...Ch. 6 - Circle the approximate part or parts of the...Ch. 6 - Prob. 6.24CPCh. 6 - This figure represents the successive ionization...Ch. 6 - In the following drawings, red spheres represent...Ch. 6 - Which of the following drawings is more likely to...Ch. 6 - Prob. 6.28CPCh. 6 - Which of the following alkali metal halides has...Ch. 6 - Which of the following alkali metal halides has...Ch. 6 - Three binary compounds are represented on the...Ch. 6 - Prob. 6.32CPCh. 6 - Prob. 6.33CPCh. 6 - What is the difference between a covalent bond and...Ch. 6 - Prob. 6.35SPCh. 6 - What is the difference between a molecule and an...Ch. 6 - Prob. 6.37SPCh. 6 - How many protons and electrons are in each of the...Ch. 6 - What is the identity of the element X in the...Ch. 6 - Prob. 6.40SPCh. 6 - Prob. 6.41SPCh. 6 - Prob. 6.42SPCh. 6 - Prob. 6.43SPCh. 6 - What doubly positive ion has the following...Ch. 6 - Prob. 6.45SPCh. 6 - Prob. 6.46SPCh. 6 - Which element in the transition-metal series Sc...Ch. 6 - Prob. 6.48SPCh. 6 - Prob. 6.49SPCh. 6 - Order the following ions from smallest to largest:...Ch. 6 - Order the following ions from smallest to largest:...Ch. 6 - Which ion has a larger atomic radius, Cu+ or Cu2+...Ch. 6 - Which ion hasa larger atomic radius, Fe2+ or Fe3+...Ch. 6 - The following ions all have the same number of...Ch. 6 - Which of the ions Se2,F,O2 and Rb+ has the largest...Ch. 6 - Which group of elements in the periodic table has...Ch. 6 - Prob. 6.57SPCh. 6 - Which element in each of the following sets has...Ch. 6 - Order the elements in each set from the smallest...Ch. 6 - (a) Which has the smaller second ionization...Ch. 6 - (a) Which has the smaller fourth ionization...Ch. 6 - Three atoms have the following electron...Ch. 6 - Three atoms have the following electron...Ch. 6 - The first four ionization energies in kJ/mol of a...Ch. 6 - The first four ionization energies in kJ/mol of a...Ch. 6 - Prob. 6.66SPCh. 6 - Prob. 6.67SPCh. 6 - Prob. 6.68SPCh. 6 - Prob. 6.69SPCh. 6 - Why is energy usually released when an electron is...Ch. 6 - Why does ionization energy increase regularly...Ch. 6 - No element has a negative second electron...Ch. 6 - Why does phosphorus have a less negative electron...Ch. 6 - Prob. 6.74SPCh. 6 - What noble-gas configurations and charge are the...Ch. 6 - Each of the following pairs of elements will react...Ch. 6 - Each of the following pairs of elements will react...Ch. 6 - Element X reacts with element Y to give a product...Ch. 6 - Element X reacts with element Y to give a product...Ch. 6 - Calculate the energy change in kilojoules per mole...Ch. 6 - Prob. 6.81SPCh. 6 - Find the lattice energy of LiBr(s) in Table 6.3,...Ch. 6 - Look up the lattice energies in Table 6.3, and...Ch. 6 - Born-4-Iaber cycles, such as those shown in...Ch. 6 - Calculate a lattice energy for CaH2(s) in...Ch. 6 - Calculate the overall energy change in kilojoules...Ch. 6 - The estimated lattice energy for CsF2(s) is +2347...Ch. 6 - Calculate the overall energy change in kilojoules...Ch. 6 - Use the data in Problem 6.88 to calculate an...Ch. 6 - Use the data and the result in Problem 6.84 to...Ch. 6 - Prob. 6.91SPCh. 6 - Calculate overall energy changes in kilojoules per...Ch. 6 - Prob. 6.93SPCh. 6 - We saw in Section 6.7 that the reaction of solid...Ch. 6 - Draw a Born—Haber cycle for the reaction of sodium...Ch. 6 - Use the following information plus the data given...Ch. 6 - Prob. 6.97SPCh. 6 - Prob. 6.98SPCh. 6 - Order the following compounds according to their...Ch. 6 - Prob. 6.100MPCh. 6 - Heating elemental cesium and platinum together for...Ch. 6 - Given the following information, construct a...Ch. 6 - Consider the electronic structure of the element...Ch. 6 - Prob. 6.104MPCh. 6 - The ionization energy of an atom can be measured...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY