
Concept explainers
(a)
Interpretation:
Electron group geometry and Molecular geometry about the central atom for
Concept Introduction:
Information about the number of bonds and types of bonds can be obtained from Lewis structure but the molecular geometry cannot be obtained. Three dimensional arrangement of atoms in a molecule can be given by molecular geometry. Physical and chemical properties are determined by the molecular geometry of the molecule.
Using VSEPR theory and Lewis structure, the molecular geometry of the molecule that contain less number of atoms can be predicted. VSEPR theory uses the information from Lewis structure of the molecule to predict the molecular geometry of the molecule. Main concept of VSEPR theory is that electron pairs that are present in the valence shell adopt arrangement in a way that minimize the repulsion between like charges.
If the central atom contains two electron pairs, then it has to be far apart means, it has to be on opposite side of the nucleus. This means the angle has to be
If the central atom contains three electron pairs, then it has to be far apart means, it has to be on corner of a triangle. This means the angle has to be
If the central atom contains four electron pairs, then it has to be far apart means, it has to be in a tetrahedral arrangement. This means the angle has to be
The collection of valence electron that is present in localized region about central atom in a molecule is known as VSEPR electron group. This may contain two electrons, four electrons, or six electrons. The electron group that contain four and six electrons repel each other.
Tetrahedral VSEPR electron group:
The four electron pairs can be of three VSEPR electron groups. They are 4 bonding electron groups, 3 bonding and 1 nonbonding electron groups, and 2 bonding and 2 nonbonding electron groups. The molecular geometry that is associated with 4 bonding electron groups is tetrahedral. The molecular geometry that is associated with 3 bonding and 1 nonbonding electron groups is trigonal pyramidal. The molecular geometry that is associated with 2 bonding and 2 nonbonding electron groups is angular.
Trigonal planar VSEPR electron group:
The three electron pairs can be of two VSEPR electron groups. They are 3 bonding electron groups, and 2 bonding and 1 nonbonding electron groups. The molecular geometry that is associated with 3 bonding electron groups is trigonal planar. The molecular geometry that is associated with 2 bonding and 1 nonbonding electron groups is angular.
Linear VSEPR electron group:
The two electron pairs can be of only one VSEPR electron groups. It is only 2 bonding electron groups and the geometry associated with it is linear geometry.
Molecules with more than one central atom:
If a molecule contains more than one central atom then the molecular geometry for each central atom is given separately following the same rules applied for the molecules that contain one central atom.
Rules for predicting Geometry of molecules:
- Lewis structure for the given molecule has to be drawn.
- The number of VSEPR electron groups present about the central atom has to be counted in Lewis structure.
- Geometry has to be assigned considering minimum repulsion between the electron groups.
(a)
Answer to Problem 5.57EP
Electron group geometry is tetrahedral and molecular geometry about the central atom in
Explanation of Solution
Given molecule is
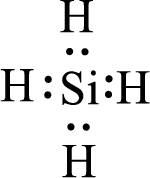
The above drawn structure for
The electron group geometry and molecular geometry about the central atom is tetrahedral.
Electron group geometry and molecular geometry is predicted for
(b)
Interpretation:
Electron group geometry and Molecular geometry about the central atom for
Concept Introduction:
Information about the number of bonds and types of bonds can be obtained from Lewis structure but the molecular geometry cannot be obtained. Three dimensional arrangement of atoms in a molecule can be given by molecular geometry. Physical and chemical properties are determined by the molecular geometry of the molecule.
Using VSEPR theory and Lewis structure, the molecular geometry of the molecule that contain less number of atoms can be predicted. VSEPR theory uses the information from Lewis structure of the molecule to predict the molecular geometry of the molecule. Main concept of VSEPR theory is that electron pairs that are present in the valence shell adopt arrangement in a way that minimize the repulsion between like charges.
If the central atom contains two electron pairs, then it has to be far apart means, it has to be on opposite side of the nucleus. This means the angle has to be
If the central atom contains three electron pairs, then it has to be far apart means, it has to be on corner of a triangle. This means the angle has to be
If the central atom contains four electron pairs, then it has to be far apart means, it has to be in a tetrahedral arrangement. This means the angle has to be
The collection of valence electron that is present in localized region about central atom in a molecule is known as VSEPR electron group. This may contain two electrons, four electrons, or six electrons. The electron group that contain four and six electrons repel each other.
Tetrahedral VSEPR electron group:
The four electron pairs can be of three VSEPR electron groups. They are 4 bonding electron groups, 3 bonding and 1 nonbonding electron groups, and 2 bonding and 2 nonbonding electron groups. The molecular geometry that is associated with 4 bonding electron groups is tetrahedral. The molecular geometry that is associated with 3 bonding and 1 nonbonding electron groups is trigonal pyramidal. The molecular geometry that is associated with 2 bonding and 2 nonbonding electron groups is angular.
Trigonal planar VSEPR electron group:
The three electron pairs can be of two VSEPR electron groups. They are 3 bonding electron groups, and 2 bonding and 1 nonbonding electron groups. The molecular geometry that is associated with 3 bonding electron groups is trigonal planar. The molecular geometry that is associated with 2 bonding and 1 nonbonding electron groups is angular.
Linear VSEPR electron group:
The two electron pairs can be of only one VSEPR electron groups. It is only 2 bonding electron groups and the geometry associated with it is linear geometry.
Molecules with more than one central atom:
If a molecule contains more than one central atom then the molecular geometry for each central atom is given separately following the same rules applied for the molecules that contain one central atom.
Rules for predicting Geometry of molecules:
- Lewis structure for the given molecule has to be drawn.
- The number of VSEPR electron groups present about the central atom has to be counted in Lewis structure.
- Geometry has to be assigned considering minimum repulsion between the electron groups.
(b)
Answer to Problem 5.57EP
Electron group geometry is tetrahedral and molecular geometry about the central atom in
Explanation of Solution
Given molecule is
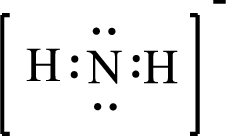
The above drawn structure for
The electron group geometry is tetrahedral and molecular geometry about the central atom is angular.
Electron group geometry and molecular geometry is predicted for
(c)
Interpretation:
Electron group geometry and Molecular geometry about the central atom for
Concept Introduction:
Information about the number of bonds and types of bonds can be obtained from Lewis structure but the molecular geometry cannot be obtained. Three dimensional arrangement of atoms in a molecule can be given by molecular geometry. Physical and chemical properties are determined by the molecular geometry of the molecule.
Using VSEPR theory and Lewis structure, the molecular geometry of the molecule that contain less number of atoms can be predicted. VSEPR theory uses the information from Lewis structure of the molecule to predict the molecular geometry of the molecule. Main concept of VSEPR theory is that electron pairs that are present in the valence shell adopt arrangement in a way that minimize the repulsion between like charges.
If the central atom contains two electron pairs, then it has to be far apart means, it has to be on opposite side of the nucleus. This means the angle has to be
If the central atom contains three electron pairs, then it has to be far apart means, it has to be on corner of a triangle. This means the angle has to be
If the central atom contains four electron pairs, then it has to be far apart means, it has to be in a tetrahedral arrangement. This means the angle has to be
The collection of valence electron that is present in localized region about central atom in a molecule is known as VSEPR electron group. This may contain two electrons, four electrons, or six electrons. The electron group that contain four and six electrons repel each other.
Tetrahedral VSEPR electron group:
The four electron pairs can be of three VSEPR electron groups. They are 4 bonding electron groups, 3 bonding and 1 nonbonding electron groups, and 2 bonding and 2 nonbonding electron groups. The molecular geometry that is associated with 4 bonding electron groups is tetrahedral. The molecular geometry that is associated with 3 bonding and 1 nonbonding electron groups is trigonal pyramidal. The molecular geometry that is associated with 2 bonding and 2 nonbonding electron groups is angular.
Trigonal planar VSEPR electron group:
The three electron pairs can be of two VSEPR electron groups. They are 3 bonding electron groups, and 2 bonding and 1 nonbonding electron groups. The molecular geometry that is associated with 3 bonding electron groups is trigonal planar. The molecular geometry that is associated with 2 bonding and 1 nonbonding electron groups is angular.
Linear VSEPR electron group:
The two electron pairs can be of only one VSEPR electron groups. It is only 2 bonding electron groups and the geometry associated with it is linear geometry.
Molecules with more than one central atom:
If a molecule contains more than one central atom then the molecular geometry for each central atom is given separately following the same rules applied for the molecules that contain one central atom.
Rules for predicting Geometry of molecules:
- Lewis structure for the given molecule has to be drawn.
- The number of VSEPR electron groups present about the central atom has to be counted in Lewis structure.
- Geometry has to be assigned considering minimum repulsion between the electron groups.
(c)
Answer to Problem 5.57EP
Electron group geometry is trigonal planar and molecular geometry about the central atom in
Explanation of Solution
Given molecule is
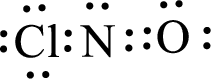
The above drawn structure for
The electron group geometry is trigonal planar and molecular geometry about the central atom is angular.
Electron group geometry and molecular geometry is predicted for
(d)
Interpretation:
Electron group geometry and Molecular geometry about the central atom for
Concept Introduction:
Information about the number of bonds and types of bonds can be obtained from Lewis structure but the molecular geometry cannot be obtained. Three dimensional arrangement of atoms in a molecule can be given by molecular geometry. Physical and chemical properties are determined by the molecular geometry of the molecule.
Using VSEPR theory and Lewis structure, the molecular geometry of the molecule that contain less number of atoms can be predicted. VSEPR theory uses the information from Lewis structure of the molecule to predict the molecular geometry of the molecule. Main concept of VSEPR theory is that electron pairs that are present in the valence shell adopt arrangement in a way that minimize the repulsion between like charges.
If the central atom contains two electron pairs, then it has to be far apart means, it has to be on opposite side of the nucleus. This means the angle has to be
If the central atom contains three electron pairs, then it has to be far apart means, it has to be on corner of a triangle. This means the angle has to be
If the central atom contains four electron pairs, then it has to be far apart means, it has to be in a tetrahedral arrangement. This means the angle has to be
The collection of valence electron that is present in localized region about central atom in a molecule is known as VSEPR electron group. This may contain two electrons, four electrons, or six electrons. The electron group that contain four and six electrons repel each other.
Tetrahedral VSEPR electron group:
The four electron pairs can be of three VSEPR electron groups. They are 4 bonding electron groups, 3 bonding and 1 nonbonding electron groups, and 2 bonding and 2 nonbonding electron groups. The molecular geometry that is associated with 4 bonding electron groups is tetrahedral. The molecular geometry that is associated with 3 bonding and 1 nonbonding electron groups is trigonal pyramidal. The molecular geometry that is associated with 2 bonding and 2 nonbonding electron groups is angular.
Trigonal planar VSEPR electron group:
The three electron pairs can be of two VSEPR electron groups. They are 3 bonding electron groups, and 2 bonding and 1 nonbonding electron groups. The molecular geometry that is associated with 3 bonding electron groups is trigonal planar. The molecular geometry that is associated with 2 bonding and 1 nonbonding electron groups is angular.
Linear VSEPR electron group:
The two electron pairs can be of only one VSEPR electron groups. It is only 2 bonding electron groups and the geometry associated with it is linear geometry.
Molecules with more than one central atom:
If a molecule contains more than one central atom then the molecular geometry for each central atom is given separately following the same rules applied for the molecules that contain one central atom.
Rules for predicting Geometry of molecules:
- Lewis structure for the given molecule has to be drawn.
- The number of VSEPR electron groups present about the central atom has to be counted in Lewis structure.
- Geometry has to be assigned considering minimum repulsion between the electron groups.
(d)
Answer to Problem 5.57EP
Electron group geometry is trigonal planar and molecular geometry about the central atom in
Explanation of Solution
Given molecule is
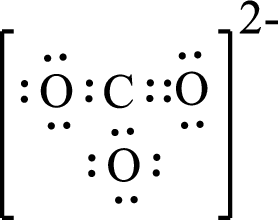
The above drawn structure for
The electron group geometry is trigonal planar and molecular geometry about the central atom is trigonal planar.
Electron group geometry and molecular geometry is predicted for
Want to see more full solutions like this?
Chapter 5 Solutions
General, Organic, and Biological Chemistry
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





