
EBK MICROBIOLOGY:W/DISEASES BY BODY...-
5th Edition
ISBN: 9780134608242
Author: BAUMAN
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 3VI
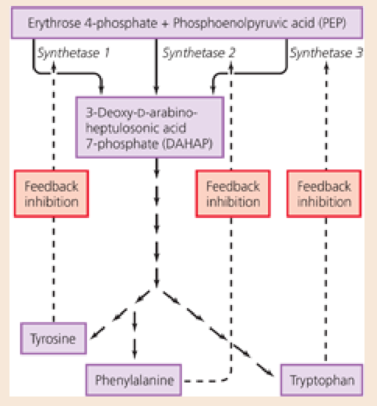
Examine the biosynthetic pathway for the production of the amino acids tryptophan, tyrosine, and phenylalanine in the figure. Where do the initial reactants (erythrose4-phosphate and PEP) originate?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why you chose this mutation. Begin by transcribing and translating BOTH the
normal and abnormal DNA sequences. The genetic code below is for your reference.
SECOND BASE OF CODON
כ
FIRST BASE OF CODON
O
THIRD BASE OF CODON
SCAGUCAGUGAGUCAG
UUU
UUC
UCU
UAU
UGU
Phenylalanine
(F)
Tyrosine (Y)
Cysteine (C)
UCC
UAC
UGC
Serine (S)
UUA
UUG
Leucine (L)
UCA
UCG_
UAA
UGA
Stop codon
-Stop codon
UAG
UGG -Tryptophan (W)
CUU
CUC
CCU
CAU
CGU
Histidine (H)
CCC
CAC
CGC
-Leucine (L)
Proline (P)
CUA
CCA
CAA
CUG
CCG
CAG-Glutamine (Q)
-Arginine (R)
CGA
CGG
AUU
ACU
AAU
AGU
AUC
Isoleucine (1)
Asparagine (N)
ACC
AAC
Threonine (T)
AUA
ACA
AAA
Methionine (M)
Lysine (K)
AUG
ACG
Start codon
AAG
AGC-Serine (S)
-Arginine (R)
AGA
AGG
GUU
GCU
GAU
GUC
GUA
GUG
GCC
Valine (V)
-Alanine (A)
GCA
GCG
GAC
GAA
GAG
Aspartic acid
(D)
GGU
Glutamic acid
(E)
GGC
GGA
GGG
Glycine (G)
In order to provide a complete answer to the question stated above, fill in the mRNA bases
and amino acid sequences by using the Genetic Code…
identify the indicated cell in white arrow
Gloeocaspa Genus - diagram a colony and label the sheath, cell wall, and cytoplasm.
Oscillatoria Genus - Diagram a trichome, and label the shealth and individual cells
Nostoc Genus- diagram a sketch of the colonoy microscopically from low power to the left of the drawing. Draw a filament showing intercalary heterocysts, and vegatative cells to the right of the drawing
Merismopedia Genus- diagram a sketch of the colony. draw and label a filament showing the colony, cell wall, and sheath.
Gloeotrichia Genus- diagram a habit sketch of the colony. draw a filament showing the heterocyst, akimetes and vegatative cells of the filament
Chapter 5 Solutions
EBK MICROBIOLOGY:W/DISEASES BY BODY...-
Ch. 5 - How can oxidation take place in an anaerobic...Ch. 5 - Why do electrons carried by NADH allow for...Ch. 5 - Why does catabolism of amino acids for energy...Ch. 5 - An uninformed student describes the Calvin-Benson...Ch. 5 - Prob. 5TMWCh. 5 - Why is feedback inhibition necessary for...Ch. 5 - Breaks a large molecule into smaller ones a....Ch. 5 - Includes dehydration synthesis reactions a....Ch. 5 - Prob. 3MCCh. 5 - Prob. 4MC
Ch. 5 - Involves the production of cell membrane...Ch. 5 - Includes hydrolytic reactions a. anabolism only b....Ch. 5 - Includes metabolism a. anabolism only b. both...Ch. 5 - Prob. 8MCCh. 5 - A reduced molecule _________. a. has gained...Ch. 5 - Prob. 10MCCh. 5 - Coenzymes are ________. a. types of apoenzymes b....Ch. 5 - Which of the following statements best describes...Ch. 5 - Which of the following does not affect the...Ch. 5 - Most oxidation reactions in bacteria involve the...Ch. 5 - Under ideal conditions, the fermentation of one...Ch. 5 - Under ideal conditions, the complete aerobic...Ch. 5 - Which of the following statements about the...Ch. 5 - Reactions involved in the light-independent...Ch. 5 - The glycolysis pathway is basically __________. a....Ch. 5 - A major difference between anaerobic respiration...Ch. 5 - 1. _______ Occurs when energy from a compound...Ch. 5 - Fill in the Blanks 1. The final electron acceptor...Ch. 5 - Fill in the Blanks 2. Two ATP molecules are used...Ch. 5 - Fill in the Blanks 3. The initial catabolism of...Ch. 5 - Fill in the Blanks 4. ________ is a cyclic series...Ch. 5 - Fill in the Blanks 5. The final electron acceptor...Ch. 5 - Fill in the Blanks 6. Three common inorganic...Ch. 5 - Fill in the Blanks 7. Anaerobic respiration...Ch. 5 - Fill in the Blanks 8. Complete the following...Ch. 5 - Prob. 9FIBCh. 5 - Fill in the Blanks 10 The main coenzymes that...Ch. 5 - VISUALIZE IT! 1 Label the mitochondrion to...Ch. 5 - Label the diagram below to indicate acetyl-CoA,...Ch. 5 - Examine the biosynthetic pathway for the...Ch. 5 - Prob. 1SACh. 5 - Why we enzymes necessary for anabolic reactions to...Ch. 5 - How do organisms control the rate of metabolic...Ch. 5 - How does a nor-competitive inhibitor at a single...Ch. 5 - Explain the mechanism of negative feedback with...Ch. 5 - Facultative anaerobes can live under either...Ch. 5 - How does oxidation of a molecule occur without...Ch. 5 - List at least four groups of microorganisms that...Ch. 5 - Why do we breathe oxygen and give of carbon...Ch. 5 - Why do cyanobacteria and algae take in carbon...Ch. 5 - What happens to the carbon atoms in sugar...Ch. 5 - How do yeast cells make alcohol and cause bread to...Ch. 5 - Where specifically does the most significant...Ch. 5 - Why are vitamins essential metabolic factors for...Ch. 5 - A laboratory scientist notices that a cer1ain...Ch. 5 - Arsenic is a poison that exists in two states in...Ch. 5 - Explain why an excess of all three of the amino...Ch. 5 - Why might an organism that uses glycolysis and the...Ch. 5 - Describe how bacterial fermentation causes milk to...Ch. 5 - Giardia intestinalis and Entamoeba histolytica are...Ch. 5 - Two cultures of a facultative anaerobe are grown...Ch. 5 - What is the maximum number of molecules of ATP...Ch. 5 - In terms of its effects on human metabolism, why...Ch. 5 - Cyanide is a potent poison because it irreversibly...Ch. 5 - How are photophosphorylation and oxidative...Ch. 5 - Members of the pathogenic bacterial genus...Ch. 5 - Compare and contrast aerobic respiration,...Ch. 5 - Scientists estimate that up to one-third of Earths...Ch. 5 - A young student was troubled by the idea that a...Ch. 5 - If a bacterium uses beta-oxidation to catabolize a...Ch. 5 - Some desert rodents rarely have water to drink....Ch. 5 - Prob. 17CTCh. 5 - We have examined the total ATP, NADH, and FADH2...Ch. 5 - Explain why hyperthermophiles do not cause disease...Ch. 5 - In addition to extremes in temperature and pH,...Ch. 5 - Figure 5.18b illustrates events in aerobic...Ch. 5 - Suppose you could insert a tiny pH probe into the...Ch. 5 - Even though Pseudomonas aeruginosa and...Ch. 5 - Photosynthetic organisms are rarely pathogenic....Ch. 5 - Prob. 25CTCh. 5 - A scientist moves a green plant grown in sunlight...Ch. 5 - What class of enzyme is involved in amination...Ch. 5 - Using the following terms, fill in the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- What Genus is this?arrow_forwardAs a medical professional, it is important to be able to discuss how genetic processes such as translation regulation can directly affect patients. Think about some situations that might involve translation regulation. Respond to the following in a minimum of 175 words: Why is translation regulation important? What are some examples of translation regulation in humans? Select one of the examples you provided and explain what happens when translation regulation goes wrong.arrow_forwardThe metabolic pathway below is used for the production of the purine nucleotides adenosine monophosphate (AMP) and guanosine monophosphate (GMP) in eukaryotic cells. Assume each arrow represents a reaction catalyzed by a different enzyme. Using the principles of feedback inhibition, propose a regulatory scheme for this pathway that ensures an adequate supply of both AMP and GMP, and prevents the buildup of Intermediates A through G when supplies of both AMP and GMP are adequate.arrow_forward
- QUESTION 27 Label the structures marked A, B, C and explain the role of structure A. W plasma membrane For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS ☐ Paragraph Π " ΩΘΗ Β Open Sans, a... 10pt EEarrow_forwardexamples of synamptomorphyarrow_forwardexamples of synamtomorphy.arrow_forward
- E. Bar Graph Use the same technique to upload the completed image. We will use a different type of graph to derive additional information from the CO2 data (Fig A1.6.2) 1. Calculate the average rate of increase in COz concentration per year for the time intervals 1959-1969, 1969- 1979, etc. and write the results in the spaces provided. The value for 1959-1969 is provided for you as an example. 2. Plot the results as a bar graph. The 1959-1969 is plotted for you. 3. Choose the graph that looks the most like yours A) E BAR GRAPH We will use a different type of graph to derive additional information from the CU, data (rig. nive). Average Yearly Rate of Observatory, Hawall interval Rate of increase per year 1959-1969 0.9 1969-1979 1979-1989 1989-1999 1999-2009 Figure A1.6.2 1999-2009 *- mrame -11- -n4 P2 جية 1989-1999 1979-1989 1969-1979 1959-1969 This bar drawn for you as an example 1.0 CO, Average Increase/Year (ppmv) B) E BAR GRAPH We will use a different type of graph to derive…arrow_forwardUse the relationships you just described to compute the values needed to fill in the blanks in the table in Fig A1.4.1 depth (a) 1.0 cml 0.7 cml cm| base dimensions (b, c)| 1.0 cm| 1.0 cm| 1.0 cm 1.0 cm| 1.0 cm| 1.0 cm volume (V) 1.0_cm' cm'| cm'| density (p) 1.0 g/cm'| 1.0 g/cm 1.0 g/cm' mass (m)| 0.3 g Column 1: depth at 1.0 cm volume mass Column 2: depth at 0.7 cm volume mass Column 3: unknown depth depth volumearrow_forwardSan Andreas Transform Boundary Plate Motion The geologic map below of southern California shows the position of the famous San Andreas Fault, a transform plate boundary between the North American Plate (east side) and the Pacific Plate (west side). The relative motion between the plates is indicated by the half arrows along the transform plate boundary (i.e., the Pacific Plate is moving to the northwest relative to the North American Plate). Note the two bodies of Oligocene volcanic rocks (labeled Ov) on the map in the previous page located along either side of the San Andreas Fault. These rocks are about 23.5 million years old and were once one body of rock. They have been separated by displacement along the fault. 21. Based on the offset of these volcanic rocks, what is the average annual rate of relative plate motion in cm/yr? SAF lab 2.jpg Group of answer choices 0.67 cm/yr 2 cm/yr 6.7 cm/yr 1.5 cm/yr CALIFORNIA Berkeley San Francisco K Os Q San Andreas Fault Ov…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning



Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY