
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
4th Edition
ISBN: 9781119761068
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.8, Problem 4.84P
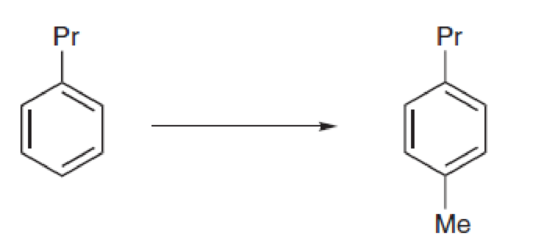
Propose an efficient synthesis for each of the following transformations.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please correct answer and don't use hand rating
Q: Draw the molecular orbital energy level diagram for the following molecules.
1- The SF4 molecule is seesaw molecular geometry and has C2v point group.
2- The Mn(CO)s molecule with C4v point group is
square pyramidal.
Please correct answer and don't use hand rating
Chapter 4 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 4.1 - Consider the following reaction, in which an...Ch. 4.1 - Prob. 4.3PCh. 4.1 - Aromatic rings will also undergo iodination when...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.3 - Prob. 4.10PCh. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.3 - Prob. 4.13P
Ch. 4.3 - Prob. 4.14PCh. 4.3 - Predict the products of the following reaction.Ch. 4.3 - Prob. 4.16PCh. 4.3 - Prob. 4.17PCh. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - Prob. 4.27PCh. 4.4 - Prob. 4.28PCh. 4.4 - And now, for a challenging problem, try to draw...Ch. 4.6 - Prob. 4.31PCh. 4.6 - Prob. 4.32PCh. 4.6 - Prob. 4.33PCh. 4.6 - Prob. 4.34PCh. 4.6 - Prob. 4.35PCh. 4.6 - Prob. 4.36PCh. 4.6 - Prob. 4.37PCh. 4.6 - Prob. 4.40PCh. 4.6 - Prob. 4.41PCh. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Prob. 4.47PCh. 4.6 - Prob. 4.48PCh. 4.6 - Prob. 4.49PCh. 4.6 - Prob. 4.50PCh. 4.6 - Prob. 4.51PCh. 4.6 - Prob. 4.52PCh. 4.6 - Prob. 4.53PCh. 4.6 - Prob. 4.54PCh. 4.6 - Prob. 4.55PCh. 4.6 - Prob. 4.56PCh. 4.7 - Prob. 4.58PCh. 4.7 - Prob. 4.59PCh. 4.7 - Prob. 4.60PCh. 4.7 - Prob. 4.61PCh. 4.7 - Prob. 4.62PCh. 4.7 - Prob. 4.63PCh. 4.7 - Prob. 4.64PCh. 4.7 - Prob. 4.65PCh. 4.7 - Prob. 4.66PCh. 4.7 - Prob. 4.67PCh. 4.7 - Can you explain why the following group is a...Ch. 4.7 - Prob. 4.70PCh. 4.7 - Prob. 4.71PCh. 4.7 - Prob. 4.72PCh. 4.7 - Prob. 4.73PCh. 4.7 - Prob. 4.74PCh. 4.7 - Prob. 4.76PCh. 4.7 - Prob. 4.77PCh. 4.7 - Prob. 4.78PCh. 4.7 - Prob. 4.79PCh. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Prob. 4.87PCh. 4.8 - Prob. 4.88PCh. 4.8 - Prob. 4.89PCh. 4.8 - Prob. 4.90PCh. 4.8 - Prob. 4.91PCh. 4.8 - Prob. 4.92PCh. 4.9 - Prob. 4.94PCh. 4.9 - Prob. 4.95PCh. 4.9 - Prob. 4.96PCh. 4.9 - Prob. 4.97PCh. 4.9 - Prob. 4.98PCh. 4.9 - Prob. 4.99PCh. 4.9 - Prob. 4.100PCh. 4.9 - Prob. 4.101PCh. 4.9 - Prob. 4.102P
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Which member of these pairs is the more polar?
(a)
(b)
(c)
Organic Chemistry
According to the logistic growth equation dNdt=rN(KN)K (A) the number of individuals added per unit time is gre...
Campbell Biology (11th Edition)
Compare enzyme induction and enzyme repression.
Microbiology: Principles and Explorations
40. Use the Henderson–Hasselbalch equation to calculate the pH of each solution.
a. a solution that is 0.145 M ...
Chemistry: A Molecular Approach (4th Edition)
3. What is free-fall, and why does it make you weightless? Briefly describe why astronauts are weightless in th...
The Cosmic Perspective (8th Edition)
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- when a 0.150 g sample of the compound was burned, it produced 0.138 g CO2 & 0.0566 g H2O. All the nitrogen in a different 0.200 g sample of the compound was converted to NH3, which was found to weigh 0.0238 g. Finally, the chlorine in a 0.125 g sample of the compound was converted to Cl- and by reacting it with AgNO3, all of the chlorine was recovered as the solid AgCl. The AgCl, when dried was found to weigh 0.251 g. What is the empirical formulaarrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forwardHow to determine if this is N- ethylsaccharin or O-ethylsaccharin or a mixture of both based on chemical shifts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY