
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
4th Edition
ISBN: 9781119761068
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4.3, Problem 4.17P
Interpretation Introduction
Interpretation:
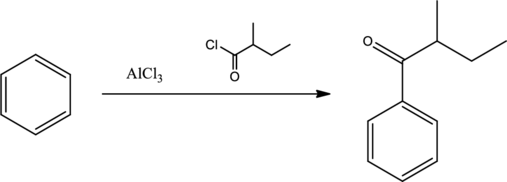
Mechanism for the given transformation has to be given.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(ii)
Draw a reasonable mechanism for the following reaction:
CI
NaOH
heat
OH
(hint: SNAr Reaction)
:
Draw the major product in each of the following reaction:
Draw the mechanism for the following Friedel-Craft reaction.
AlBr3
Br
Chapter 4 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 4.1 - Consider the following reaction, in which an...Ch. 4.1 - Prob. 4.3PCh. 4.1 - Aromatic rings will also undergo iodination when...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.3 - Prob. 4.10PCh. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.3 - Prob. 4.13P
Ch. 4.3 - Prob. 4.14PCh. 4.3 - Predict the products of the following reaction.Ch. 4.3 - Prob. 4.16PCh. 4.3 - Prob. 4.17PCh. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - Prob. 4.27PCh. 4.4 - Prob. 4.28PCh. 4.4 - And now, for a challenging problem, try to draw...Ch. 4.6 - Prob. 4.31PCh. 4.6 - Prob. 4.32PCh. 4.6 - Prob. 4.33PCh. 4.6 - Prob. 4.34PCh. 4.6 - Prob. 4.35PCh. 4.6 - Prob. 4.36PCh. 4.6 - Prob. 4.37PCh. 4.6 - Prob. 4.40PCh. 4.6 - Prob. 4.41PCh. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Prob. 4.47PCh. 4.6 - Prob. 4.48PCh. 4.6 - Prob. 4.49PCh. 4.6 - Prob. 4.50PCh. 4.6 - Prob. 4.51PCh. 4.6 - Prob. 4.52PCh. 4.6 - Prob. 4.53PCh. 4.6 - Prob. 4.54PCh. 4.6 - Prob. 4.55PCh. 4.6 - Prob. 4.56PCh. 4.7 - Prob. 4.58PCh. 4.7 - Prob. 4.59PCh. 4.7 - Prob. 4.60PCh. 4.7 - Prob. 4.61PCh. 4.7 - Prob. 4.62PCh. 4.7 - Prob. 4.63PCh. 4.7 - Prob. 4.64PCh. 4.7 - Prob. 4.65PCh. 4.7 - Prob. 4.66PCh. 4.7 - Prob. 4.67PCh. 4.7 - Can you explain why the following group is a...Ch. 4.7 - Prob. 4.70PCh. 4.7 - Prob. 4.71PCh. 4.7 - Prob. 4.72PCh. 4.7 - Prob. 4.73PCh. 4.7 - Prob. 4.74PCh. 4.7 - Prob. 4.76PCh. 4.7 - Prob. 4.77PCh. 4.7 - Prob. 4.78PCh. 4.7 - Prob. 4.79PCh. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Prob. 4.87PCh. 4.8 - Prob. 4.88PCh. 4.8 - Prob. 4.89PCh. 4.8 - Prob. 4.90PCh. 4.8 - Prob. 4.91PCh. 4.8 - Prob. 4.92PCh. 4.9 - Prob. 4.94PCh. 4.9 - Prob. 4.95PCh. 4.9 - Prob. 4.96PCh. 4.9 - Prob. 4.97PCh. 4.9 - Prob. 4.98PCh. 4.9 - Prob. 4.99PCh. 4.9 - Prob. 4.100PCh. 4.9 - Prob. 4.101PCh. 4.9 - Prob. 4.102P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardConsider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forward
- In a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardGiven the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forwardk₁ Given the reaction R₂ R + R, indicate k-1 (A). the rate law with respect to R2: (B). the rate law with respect to R: d[R₂] dt d[R] dtarrow_forward
- Given the reaction R+ Q → P, indicate (A). the rate law with respect to P: (B). the rate law with respect to R: (C). the rate law with respect to Q: d[P] dt d[R] dt d[Q] dtarrow_forwardThe reaction for obtaining NO2 from NO and O2 has the rate equation: v = k[NO]2[O2]. Indicate which of the following options is correct.(A). This rate equation is inconsistent with the reaction consisting of a single trimolecular step.(B). Since the overall order is 3, the reaction must necessarily have some trimolecular step in its mechanism.(C). A two-step mechanism: 1) NO + NO ⇄ N2O2 (fast); 2) N2O2 + O2 → NO2 + NO2 (slow).(D). The mechanism must necessarily consist of three unimolecular elementary steps with very similar rate constants.arrow_forwarda. What is the eluent used in the column chromatography here (a “silica plug filtration” is essentially a very short column)? b. The spectroscopy of compound 5b is described in the second half of this excerpt, including 1H-NMR and 13C-NMR (which you will learn about in CHEM 2412L), MS (which you will learn about later in CHEM 2411L) and IR. One of the IR signals is at 3530 cm-1. What functional group does this indicate might be present in compound 5b?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT