
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
4th Edition
ISBN: 9781119761068
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.3, Problem 4.15P
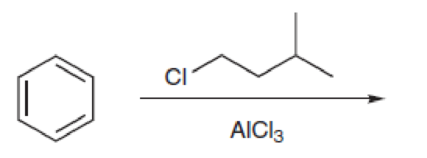
Predict the products of the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An einstein is the amount of energy needed to dissociate 1 mole of a substance. If we have 0.58 moles, do we need 0.58 einsteins to dissociate that substance?
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.
Chapter 4 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 4.1 - Consider the following reaction, in which an...Ch. 4.1 - Prob. 4.3PCh. 4.1 - Aromatic rings will also undergo iodination when...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.3 - Prob. 4.10PCh. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.3 - Prob. 4.13P
Ch. 4.3 - Prob. 4.14PCh. 4.3 - Predict the products of the following reaction.Ch. 4.3 - Prob. 4.16PCh. 4.3 - Prob. 4.17PCh. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - Prob. 4.27PCh. 4.4 - Prob. 4.28PCh. 4.4 - And now, for a challenging problem, try to draw...Ch. 4.6 - Prob. 4.31PCh. 4.6 - Prob. 4.32PCh. 4.6 - Prob. 4.33PCh. 4.6 - Prob. 4.34PCh. 4.6 - Prob. 4.35PCh. 4.6 - Prob. 4.36PCh. 4.6 - Prob. 4.37PCh. 4.6 - Prob. 4.40PCh. 4.6 - Prob. 4.41PCh. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Prob. 4.47PCh. 4.6 - Prob. 4.48PCh. 4.6 - Prob. 4.49PCh. 4.6 - Prob. 4.50PCh. 4.6 - Prob. 4.51PCh. 4.6 - Prob. 4.52PCh. 4.6 - Prob. 4.53PCh. 4.6 - Prob. 4.54PCh. 4.6 - Prob. 4.55PCh. 4.6 - Prob. 4.56PCh. 4.7 - Prob. 4.58PCh. 4.7 - Prob. 4.59PCh. 4.7 - Prob. 4.60PCh. 4.7 - Prob. 4.61PCh. 4.7 - Prob. 4.62PCh. 4.7 - Prob. 4.63PCh. 4.7 - Prob. 4.64PCh. 4.7 - Prob. 4.65PCh. 4.7 - Prob. 4.66PCh. 4.7 - Prob. 4.67PCh. 4.7 - Can you explain why the following group is a...Ch. 4.7 - Prob. 4.70PCh. 4.7 - Prob. 4.71PCh. 4.7 - Prob. 4.72PCh. 4.7 - Prob. 4.73PCh. 4.7 - Prob. 4.74PCh. 4.7 - Prob. 4.76PCh. 4.7 - Prob. 4.77PCh. 4.7 - Prob. 4.78PCh. 4.7 - Prob. 4.79PCh. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Prob. 4.87PCh. 4.8 - Prob. 4.88PCh. 4.8 - Prob. 4.89PCh. 4.8 - Prob. 4.90PCh. 4.8 - Prob. 4.91PCh. 4.8 - Prob. 4.92PCh. 4.9 - Prob. 4.94PCh. 4.9 - Prob. 4.95PCh. 4.9 - Prob. 4.96PCh. 4.9 - Prob. 4.97PCh. 4.9 - Prob. 4.98PCh. 4.9 - Prob. 4.99PCh. 4.9 - Prob. 4.100PCh. 4.9 - Prob. 4.101PCh. 4.9 - Prob. 4.102P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Exercise 2
Calculate the pH of a solution containing 0.085 M nitrous acid (HNO2, Ka = 4.5 x 10-4) an...
Chemistry: The Central Science (14th Edition)
PRACTICE PROBLEM 18.10 How would you use the acetoacetic ester synthesis to prepare the following?
Organic Chemistry
In a single-slit diffraction experiment, what must be the ratio of the slit width to the wavelength if the seco...
Fundamentals of Physics Extended
How does an obligate aerobe differ from a facultative aerobe?
Brock Biology of Microorganisms (15th Edition)
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
4. How do gross anatomy and microscopic anatomy differ?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY