
Introductory Chemistry: A Foundation
8th Edition
ISBN: 9781285199030
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 50QAP
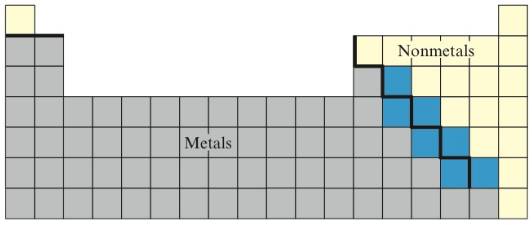
The elements that lie close to the “stair-step” line as shown below in blue are called
.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
a. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression:
AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T
A. Calculate the value of AS for the process.
B. Next, use the Gibbs-Helmholtz equation:
(a(AG/T))
ΔΗ
-
T2
to calculate the value of AH for the process.
None
None
Chapter 4 Solutions
Introductory Chemistry: A Foundation
Ch. 4.4 - Exercise 4.1 Write the formula for each of the...Ch. 4.5 - The average diameter of an atom ¡s1.31010 m. What...Ch. 4.5 - You have learned about three different models of...Ch. 4.7 - Exercise 4.2 Give the number of protons, neutrons,...Ch. 4.7 - Exercise 4.3 Give the number of protons, neutrons,...Ch. 4.7 - Exercise 4.4 Give the symbol for the phosphorus...Ch. 4.8 - Exercise 4.5 Give the symbol and atomic number for...Ch. 4.11 - Thomson and Rutherford helped to show that atoms...Ch. 4.11 - Prob. 4.6SCCh. 4 - Knowing the number of protons in the atom of a...
Ch. 4 - The average mass of a carbon atom is 12.011....Ch. 4 - Prob. 3ALQCh. 4 - Prob. 4ALQCh. 4 - The vitamin niacin (nicotinic acid,C6H5NO2 ) can...Ch. 4 - One of the best indications of a useful theory is...Ch. 4 - Prob. 7ALQCh. 4 - How does Dalton’s atomic theory account for the...Ch. 4 - Prob. 9ALQCh. 4 - These questions concern the work of J. J. Thomson:...Ch. 4 - Heat is applied to an ice cube until only steam is...Ch. 4 - What makes a carbon atom different from a nitrogen...Ch. 4 - Hundreds of years ago, alchemists tried to turn...Ch. 4 - Chlorine has two prominent isotopes,37Cl and35Cl ....Ch. 4 - Prob. 15ALQCh. 4 - Prob. 16ALQCh. 4 - Rutherford was surprised when some of theparticles...Ch. 4 - It is good practice to actively read the textbook...Ch. 4 - Why is the term “sodium chloride molecule”...Ch. 4 - Both atomic elements and molecular elements exist....Ch. 4 - Now that you have gone through Chapter 4, go back...Ch. 4 - Write the formula for each of the following...Ch. 4 - Prob. 23ALQCh. 4 - What were the four fundamental substances...Ch. 4 - Prob. 2QAPCh. 4 - Prob. 3QAPCh. 4 - Prob. 4QAPCh. 4 - What are the live most abundant elements (by mass)...Ch. 4 - Read the “Chemistry in Focus” segment Trace...Ch. 4 - Prob. 7QAPCh. 4 - The symbols for most elements are based on the...Ch. 4 - Find the symbol in Column 2 for each name in...Ch. 4 - Prob. 10QAPCh. 4 - Use the periodic table shown in Fig. 4.9 to find...Ch. 4 - Prob. 12QAPCh. 4 - Prob. 13QAPCh. 4 - Prob. 14QAPCh. 4 - A given compound always contains the same...Ch. 4 - Correct each of the following misstatements from...Ch. 4 - Prob. 17QAPCh. 4 - A given compound always contains the same relative...Ch. 4 - Based on the following word descriptions, write...Ch. 4 - Based on the following word descriptions, write...Ch. 4 - Scientists J. J. Thomson and William Thomson (Lord...Ch. 4 - True or false? Rutherford’s bombardment...Ch. 4 - Where are neutrons found in an atom? Are neutrons...Ch. 4 - What are the positively charged particles found in...Ch. 4 - Do the proton and the neutron have exactly the...Ch. 4 - The proton and the (electron/neutron) have almost...Ch. 4 - An average atomic nucleus has a diameter of about...Ch. 4 - Prob. 28QAPCh. 4 - Prob. 29QAPCh. 4 - True or false? The mass number of a nucleus...Ch. 4 - For an isolated atom, why do we expect the number...Ch. 4 - Why do we not necessarily expect the number of...Ch. 4 - Dalton’s original atomic theory proposed that all...Ch. 4 - Prob. 34QAPCh. 4 - For each of the following elements, use the...Ch. 4 - Prob. 36QAPCh. 4 - Write the atomic symbol(ZAX) for each of the...Ch. 4 - Write the atomic symbol(ZAX) for each of the...Ch. 4 - How many protons and neutrons are contained in the...Ch. 4 - Read the Chemistry in Focus” segment “Whair”Do You...Ch. 4 - Read the “Chemistry in Focus” segmentIsotope...Ch. 4 - Complete the following table. Name Symbol Atomic...Ch. 4 - True or false? The elements are arranged in the...Ch. 4 - Prob. 44QAPCh. 4 - List the characteristic physical properties that...Ch. 4 - Where are the metallic elements found on the...Ch. 4 - Prob. 47QAPCh. 4 - List five nonmetallic elements that exist as...Ch. 4 - Under ordinary conditions, only a few pure...Ch. 4 - The elements that lie close to the “stair-step”...Ch. 4 - Prob. 51QAPCh. 4 - Without looking at your textbook or the periodic...Ch. 4 - Prob. 53QAPCh. 4 - Prob. 54QAPCh. 4 - Most substances are composed of _________ rather...Ch. 4 - Prob. 56QAPCh. 4 - Prob. 57QAPCh. 4 - Prob. 58QAPCh. 4 - Molecules of nitrogen gas and oxygen gas are said...Ch. 4 - Give three examples of gaseous elements that exist...Ch. 4 - Prob. 61QAPCh. 4 - If sodium chloride (table salt) is melted and then...Ch. 4 - Prob. 63QAPCh. 4 - The two most common elemental forms of carbon are...Ch. 4 - An isolated atom has a net charge of ________ .Ch. 4 - Prob. 66QAPCh. 4 - A simple ion with a 3+ charge (for example, A13+)...Ch. 4 - An ion that has two more electrons outside the...Ch. 4 - Prob. 69QAPCh. 4 - Prob. 70QAPCh. 4 - Prob. 71QAPCh. 4 - True or false?N3 andP3 contain a different number...Ch. 4 - How many electrons are present in each of the...Ch. 4 - Prob. 74QAPCh. 4 - For the following processes that show the...Ch. 4 - For the following ions, indicate whether electrons...Ch. 4 - For each of the following atomic numbers, use the...Ch. 4 - On the basis of the element’s location in the...Ch. 4 - List some properties of a substance that would...Ch. 4 - Prob. 80QAPCh. 4 - Prob. 81QAPCh. 4 - Prob. 82QAPCh. 4 - For each of the following positive ions, use the...Ch. 4 - For each of the following negative ions, use the...Ch. 4 - Prob. 85APCh. 4 - Prob. 86APCh. 4 - Prob. 87APCh. 4 - Which of the following is/are true regarding the...Ch. 4 - Prob. 89APCh. 4 - Which subatomic particles contribute most to the...Ch. 4 - Is it possible for the same Iwo elements to form...Ch. 4 - Carbohydrates, a class of compounds containing the...Ch. 4 - Prob. 93APCh. 4 - How many protons and neutrons are contained in the...Ch. 4 - Though the common isotope of aluminum has a mass...Ch. 4 - Prob. 96APCh. 4 - Prob. 97APCh. 4 - What is the symbol for an ion with a 1 — charge....Ch. 4 - Prob. 99APCh. 4 - Prob. 100APCh. 4 - Prob. 101APCh. 4 - A metal ion with a 2+ charge contains 34 neutrons...Ch. 4 - Prob. 103APCh. 4 - Write the simplest formula for each of the...Ch. 4 - Prob. 105APCh. 4 - Write the atomic symbol(ZAX) for each of the...Ch. 4 - How many protons and neutrons are contained in the...Ch. 4 - Prob. 108APCh. 4 - Prob. 109APCh. 4 - Prob. 110APCh. 4 - Prob. 111CPCh. 4 - Prob. 112CPCh. 4 - Complete the following table. Number of Protons...Ch. 4 - Prob. 114CPCh. 4 - Using the periodic table, complete the following...Ch. 4 - Prob. 116CPCh. 4 - Which of the following is(are) correct? a.40Ca2+...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forwardPlease help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forward
- Which of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY