
Concept explainers
Interpretation:
Whether the given statement is valid or not should be determined.
Concept Introduction:
The ionization of the elements present in a compound depends upon several factors. The ionization is parallel to the polarization thus a more polarize molecule leads to ionic compound i.e. permanent presence of two ions in the compound which is pictorial can be shown as:
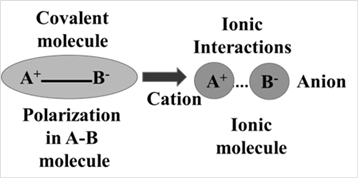
Thus more polarisability in the molecule leads to the formation of an ionic compound and vice-versa. The attaining covalent character by ionic compounds as a consequence of polarization may be generalized in terms of Fazan’s rules. According to which an ion will form more easily when-
(i) The electronic structure of the ion is stable.
(ii) The charge on the ion is small.
(iii) A small atom forms the anion and a large atom forms the cation.
Thus the covalent character in a molecule will be favorable when-
(i) The ions have high charge
(ii) The small size of the cation and large size of the anion.
(iii) The cations with 18 electrons structures.
(iv) The compound will be less soluble in a polar solvent like water.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry For Changing Times (14th Edition)
- Please provide with answer, steps and explanation of ideas to solve.arrow_forwardUsing what we have learned in CHEM 2310 and up through class on 1/31, propose a series of reaction steps to achieve the transformation below. Be sure to show all reagents and intermediates for full credit. You do not need to draw mechanism arrows, but you do need to include charges where appropriate. If you do not put your group name, you will get half credit at most. ? Brarrow_forwardDraw a mechanism for the formation of 2-bromovanillin using bromonium ion as the reactive electrophile.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





