
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4, Problem 4.27UKC
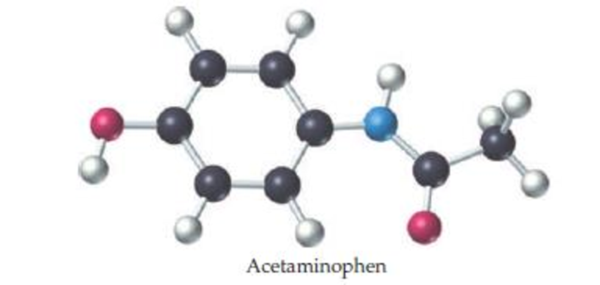
The ball-and-stick molecular model shown here is a representation of acetaminophen, the active ingredient in over-the-counter headache remedies such as Tylenol. The lines indicate only the connections between atoms not whether the bonds are single, double, or triple (red = O, gray = C, blue = N, ivory = H).
- (a) What is the molecular formula of acetaminophen?
- (b) Indicate the positions of the multiple bonds in acetaminophen.
- (c) What is the geometry around each carbon and each nitrogen?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Here is my literature Beta Carotene HPLC analysis graph.
Can you help me explain what each peak is at each retention time?
Thank You :D
I have a literature B-Carotene HPLC graph in which showcases a retention time of roughly 23.6 and 25.1.
Please help me compare my two different Anti-Oxidant Juice graphs. (Attached)
The juices provided are: V8 Carrot Ginger Blend and V8 Original Blend
Noticing the HPLC graphs I saw no peaks for the Original Blend for B-Carotene.
However the Carrot Ginger Blend showed similar peaks --> Why is this reason?
Please explain in terms of Retention time and Area (Under Curve).
Thank You!
Calculate pH of a solution prepared by dissolving 1.60g of sodium acetate, in 88.5 mL of 0.10 M acetic acid. Assume the volume change upon dissolving the sodium acetate is negligible. Ka is 1.75 x 10^-5
Chapter 4 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 4.1 - Prob. 4.1PCh. 4.2 - Prob. 4.2PCh. 4.2 - Prob. 4.3PCh. 4.3 - Prob. 4.4PCh. 4.3 - Prob. 4.5PCh. 4.4 - The BF3 molecule can also react with NH3 by...Ch. 4.5 - Prob. 4.7PCh. 4.7 - Prob. 4.8PCh. 4.7 - Add lone pairs where appropriate to the following...Ch. 4.7 - Prob. 4.10P
Ch. 4.7 - Prob. 4.11PCh. 4.7 - The molecular model shown here is a representation...Ch. 4.7 - Prob. 4.1CIAPCh. 4.7 - Prob. 4.2CIAPCh. 4.7 - Prob. 4.13PCh. 4.8 - Prob. 4.3CIAPCh. 4.8 - Prob. 4.4CIAPCh. 4.8 - Prob. 4.14PCh. 4.8 - Prob. 4.15PCh. 4.8 - Prob. 4.16PCh. 4.8 - Prob. 4.17KCPCh. 4.9 - The elements H, N, O, P, and S are commonly bonded...Ch. 4.9 - Prob. 4.19PCh. 4.10 - Look at the molecular shape of formaldehyde (CH2O)...Ch. 4.10 - Prob. 4.21PCh. 4.10 - Prob. 4.22KCPCh. 4.11 - Prob. 4.5CIAPCh. 4.11 - Prob. 4.6CIAPCh. 4.11 - Prob. 4.23PCh. 4.11 - Prob. 4.24PCh. 4 - What is the geometry around the central atom in...Ch. 4 - Prob. 4.26UKCCh. 4 - The ball-and-stick molecular model shown here is a...Ch. 4 - Prob. 4.28UKCCh. 4 - Prob. 4.29UKCCh. 4 - Prob. 4.30UKCCh. 4 - What is a covalent bond, and how does it differ...Ch. 4 - Prob. 4.32APCh. 4 - When are multiple bonds formed between atoms and...Ch. 4 - Identify the bonds formed between the following...Ch. 4 - Prob. 4.35APCh. 4 - Prob. 4.36APCh. 4 - Prob. 4.37APCh. 4 - Prob. 4.38APCh. 4 - Prob. 4.39APCh. 4 - Prob. 4.40APCh. 4 - Prob. 4.41APCh. 4 - Prob. 4.42APCh. 4 - Prob. 4.43APCh. 4 - Prob. 4.44APCh. 4 - Prob. 4.45APCh. 4 - Prob. 4.46APCh. 4 - Prob. 4.47APCh. 4 - If a research paper appeared reporting the...Ch. 4 - Consider the following possible structural...Ch. 4 - Prob. 4.50APCh. 4 - Prob. 4.51APCh. 4 - Prob. 4.52APCh. 4 - Prob. 4.53APCh. 4 - Prob. 4.54APCh. 4 - Draw a Lewis structure for the following...Ch. 4 - Prob. 4.56APCh. 4 - Ethanol, or grain alcohol, has the formula C2H6O...Ch. 4 - Prob. 4.58APCh. 4 - Tetrachloroethylene, C2Cl4, is used commercially...Ch. 4 - Prob. 4.60APCh. 4 - The carbonate ion, CO32, contains a double bond....Ch. 4 - Prob. 4.62APCh. 4 - Prob. 4.63APCh. 4 - Prob. 4.64APCh. 4 - Prob. 4.66APCh. 4 - Predict the geometry around each carbon atom in...Ch. 4 - Prob. 4.68APCh. 4 - Prob. 4.69APCh. 4 - Prob. 4.70APCh. 4 - Prob. 4.71APCh. 4 - Prob. 4.72APCh. 4 - Which of the following bonds are polar? If a bond...Ch. 4 - Prob. 4.74APCh. 4 - Based on electronegativity differences, would you...Ch. 4 - Arrange the following molecules in order of the...Ch. 4 - Prob. 4.77APCh. 4 - Prob. 4.78APCh. 4 - Prob. 4.79APCh. 4 - Prob. 4.80APCh. 4 - Prob. 4.81APCh. 4 - Prob. 4.82APCh. 4 - Prob. 4.83APCh. 4 - Prob. 4.84APCh. 4 - Prob. 4.85CPCh. 4 - Prob. 4.86CPCh. 4 - Prob. 4.87CPCh. 4 - Prob. 4.88CPCh. 4 - Prob. 4.89CPCh. 4 - The phosphonium ion, PH4+, is formed by reaction...Ch. 4 - Prob. 4.91CPCh. 4 - Prob. 4.92CPCh. 4 - Prob. 4.93CPCh. 4 - Prob. 4.94CPCh. 4 - Prob. 4.95CPCh. 4 - Prob. 4.96CPCh. 4 - Prob. 4.97CPCh. 4 - Write Lewis structures for molecules with the...Ch. 4 - Prob. 4.99CPCh. 4 - Prob. 4.100GPCh. 4 - Hydrazine is a substance used to make rocket fuel....Ch. 4 - Prob. 4.102GPCh. 4 - Titanium forms both molecular and ionic compounds...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Show a mechanism that leads to the opening of the ring below under acid-catalyzed conditions. Give the correct Fischer projection for this sugar.arrow_forwardWhat is the stereochemical relationship between B & C?arrow_forwardDon't use ai or any chat gpt will dislike okk just use accurate information okkk okkk just solve full accurate. don't use guidelines okk just did it accurate 100% sure experts solve it correct complete solutions okkk follow all instructions requirements okkkarrow_forward
- Problem 15 of 15 Submit Using the following reaction data points, construct Lineweaver-Burk plots for an enzyme with and without an inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. Using the information from this plot, determine the type of inhibitor present. 1 mM-1 1 s mM -1 [S]' V' with 10 μg per 20 54 10 36 20 5 27 2.5 23 1.25 20 Answer: |||arrow_forward12:33 CO Problem 4 of 15 4G 54% Done On the following Lineweaver-Burk -1 plot, identify the by dragging the Km point to the appropriate value. 1/V 40 35- 30- 25 20 15 10- T Км -15 10 -5 0 5 ||| 10 15 №20 25 25 30 1/[S] Г powered by desmosarrow_forward1:30 5G 47% Problem 10 of 15 Submit Using the following reaction data points, construct a Lineweaver-Burk plot for an enzyme with and without a competitive inhibitor by dragging the points to their relevant coordinates on the graph and drawing a line of best fit. 1 -1 1 mM [S]' s mM¹ with 10 mg pe 20 V' 54 10 36 > ст 5 27 2.5 23 1.25 20 Answer: |||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning



Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license