
(Sample Problems 4.1 and 4.2)
4.1 What two factors cause water to be polar?
Interpretation: Two factors that cause polarity in
Concept introduction: Solution is made by two portions, solute, and solvent. Solute represents substance present in small quantity while solvent represents substance that dissolves solute.
Polarity in molecule is generated if it has unequal distributed electron density between bonds. Polar molecule has partial charges on atoms of the molecule. The general representation of polar molecule is as follows:
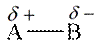
Here, B has high electronegative value than A.
Explanation of Solution
Water molecule has bend shape. In this shape, oxygen present as central atom and two hydrogen atoms are present as side atom. Structure of

Bond
Want to see more full solutions like this?
Chapter 4 Solutions
Principles of General Chemistry
- Calculate the stoichiometric amount of CaCl2 needed to convert all of the CuSO4 into CuCl2.arrow_forwardH CH تنی Cl 1. NaCN, DMF 2. LIAIH4, ether H₂O pyridine N NH₂ 5 CH H 1 HNO, H₂SO 2. Nal NH2 Br Br HNO₂ CuCl H₂SO HCI CH3 H3C NN HSO KCN CuCN 1. HNO₂, H₂SO O₂N NH2 2. OH ཀ་ལས། །ས་ཅན་ :i་དེ་མ་མ་སེ་ NH₂ CH3 1. HNO₂, H₂SO4 2. H3PO₂ 1 HNO2, H2SO4 2. Nalarrow_forwardive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forward
- If a pharmacy chain sold 65 million 500-mg tablets of aspirin, how many US tons of aspirin does this represent? Report your answer to 2 significant figures.arrow_forwardHere are the options: reducing a monosaccharide a non reducing disaccharide amylopectin cellulose 1,4' beta- glycosidearrow_forwardRefer to the monosaccharides below to answer each of the following questions: CH2OH CHO CH₂OH CHZOH 0 H OH 0 0 HO H H OH HO H HO H H OH HO H CHZOH H OH HO H HO H CHZOH CHZOH CH3 a Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. A. Xylulose is B. Erythrulose is C. Sorbose is D. Rhamnose isarrow_forward
- Refer to the sugars below to answer the following questions. Choose the sugar that best fits each escription and place the letter of the sugar in the blank to the left of the description. There is only one orrect answer for each question, but sugars may be used more than once. CH₂OH 0 CHO HO H CHO CH₂OH HO H HO H HO H H OH HH OH OH H OH H OH HO H CH₂OH H OH CH₂OH CH₂OH CH₂OH a (-)-tagatose b. (+) gulose c. (-)-erythrose d (-)-n bulos A. ARCD a D-ketohexose B. C. D. oxidizes to an optically inactive aldaric acid a dextrorotary hexose a ketose with two chirality centersarrow_forwardDraw the structure of the aldol, self condensation product for each of the following compounds if a compound does not undergo aldol self condensation explain why it does notarrow_forwardShow how each of the following transformations might be best accomplished. More than one step may required. Show all reagents and all intermediate structures. [4 only] CH3 A. CH CH2 C Br CH3 B OH only source of carbon CH3 CH CH2 C NHz CH 3 Harrow_forward
- . Choose a structure from the list provided below that best fits each of the following escriptions. Place the letter of the structure in the blank to the left of the description. There is nly one correct answer for each question. starch HO CH₂OH b. cellulose d. CH₂OH HO OH HO HO OH OH OH f. sucrose CH₂OH OH OH HO OCH₂ OH a monosaccharide that gives a negative Benedict's Test. a ẞ-1,4'-glycoside a disaccharidearrow_forwardShow how each of the following transformations might be best accomplished. More than one step may required. Show all reagents and all intermediate structures. [4 only] CH3 A. CH CH2 C Br CH3 CH3 CH3CH2 C NH2 CH3 B OH any source of carbon N MIHarrow_forwardConsider the reaction below to answer the following questions. 0 0 25 PS ES 1919sds-III msx H H + 5% NaOCH 3, CH3OHA O CH₂OH Jeiniog 2E1 gniwool of mor]. Ignibuloni 9vil 19 A B 11 >buoqm gniwollol so dass 101 tomboy boo-11Coble or to r ton auch i viw ninlaxs, noitsausbroo 152 lobla ogsbau ton 250b br A. Which carbonyl compound functions as the electrophile in this reaction? B. Draw the structure of the enolate ion that is generated during the course of this reaction. C. This reaction is an example of: a. a mixed Claisen condensation. b. C. d. a Dieckman condensation. a Michael reaction. a mixed aldol reaction. HD HDarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



