
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 29P
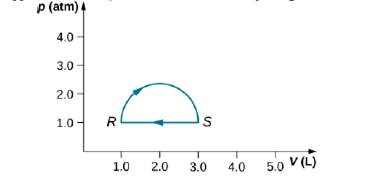
(a) Calculate the work done by the gas along the closed path shown below. The curved section between R and S is semicircular. (b) If the process is carried out in the opposite direction, what is the work done by the gas?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Determine the shear and moment diagram for the beam shown in Fig.1.
A
2 N/m
10 N
8 N
6 m
B
4m
Fig.1
40 Nm
Steps:
1) Determine the reactions at the fixed support (RA and MA) (illustrated
in Fig 1.1)
2) Draw the free body diagram on the first imaginary cut (fig. 1.2), and
determine V and M.
3) Draw the free body diagram on the second imaginary cut (fig. 1.3),
and determine V and M.
4) Draw the shear and moment diagram
Considering the cross-sectional area shown in Fig.2:
1. Determine the coordinate y of the centroid G (0, ỹ).
2. Determine the moment of inertia (I).
3. Determine the moment of inertia (Ir) (with r passing through G and
r//x (// parallel).
4 cm
28 cm
G3+
G
4 cm
y
12 cm
4 cm
24 cm
x
I need help understanding 7.
Chapter 3 Solutions
University Physics Volume 2
Ch. 3 - The paths ABC, AC, and ADC represent three...Ch. 3 - Check Your Understanding The quantities below...Ch. 3 - Check Your Understanding Why was it necessary to...Ch. 3 - Check Your Understanding When 1.00 g of ammonia...Ch. 3 - Consider these scenarios and state whether work is...Ch. 3 - Is it possible to determine whether a change in...Ch. 3 - When a liquid is vaporized, its change in internal...Ch. 3 - Why does a bicycle pump feel warm as you inflate...Ch. 3 - Is it possible for the temperature of a system to...Ch. 3 - What does the first law of thermodynamics tell us...
Ch. 3 - Does adding heat to a system always increase its...Ch. 3 - A great deal of effort, time, and money has been...Ch. 3 - When a gas expands isothermally, it does work....Ch. 3 - If the pressure and volume of a system are given,...Ch. 3 - It is unlikely that a process can be isothermal...Ch. 3 - How can an object transfer heat if the object does...Ch. 3 - Most materials expand when heated. One notable...Ch. 3 - Why are there two specific heats for gases Cp and...Ch. 3 - Is it possible for to be smaller than unity? `Ch. 3 - Would you expect to be larger for a gas or a...Ch. 3 - There is no change in the internal of an ideal gas...Ch. 3 - Does a gas do any work when it expands...Ch. 3 - A gas follows on an isothermal curve, where p is...Ch. 3 - A mole of gas has isobaric expansion coefficient...Ch. 3 - Find the equation of state of a solid that has an...Ch. 3 - A gas at a pressure of 2.00 atm undergoes a...Ch. 3 - It takes 500 J of work to compress...Ch. 3 - It is found that, when a dilute gas expands...Ch. 3 - In a quasi-static isobaric expansion. 500 J of...Ch. 3 - When a gas undergoes a quasi-static isobaric...Ch. 3 - An ideal gas expands quasi-statically and...Ch. 3 - As shown below, calculate the work done by the gas...Ch. 3 - (a) Calculate the work done by the gas along the...Ch. 3 - An ideal gas expands quasi-statically to three...Ch. 3 - A dilute gas at a pressure of 2.0 atm and a volume...Ch. 3 - What is the average mechanical energy of the atoms...Ch. 3 - What is the internal energy of 6.00 mol of an...Ch. 3 - Calculate the internal energy of 15 mg of helium...Ch. 3 - Two monatomic ideal gases A and B are at the same...Ch. 3 - The van der Waals coefficients for oxygen are...Ch. 3 - Find the work done in the quasi-static processes...Ch. 3 - When a dilute gas expands quasi-statically from...Ch. 3 - In a quasi-static isobaric expansion, 500 J of...Ch. 3 - An ideal gas quasi-statically and isothermally...Ch. 3 - As shown below, if the heat absorbed by the gas...Ch. 3 - During the isobaric expansion from A to B...Ch. 3 - (a) What is the change in internal energy for the...Ch. 3 - When a gas expands along path AC shown below, it...Ch. 3 - When a gas expands along AB (see below), it does...Ch. 3 - A dilute gas is stored in the left chamber of a...Ch. 3 - Ideal gases A and B are stored in the left and...Ch. 3 - An ideal monatomic gas at a pressure of 2.0105N/m2...Ch. 3 - Consider the process for steam in a cylinder shown...Ch. 3 - The state of 30 moles of steam in a cylinder is...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - A metallic container of fixed volume of 2.5103 m3...Ch. 3 - A gas in a cylindrical closed container is...Ch. 3 - Two moles of a monatomic ideal gas at (5 MPa, 5 L)...Ch. 3 - Consider a transformation from point A to B in a...Ch. 3 - Consider a cylinder with a movable piston...Ch. 3 - An ideal gas expands isothermally along AB and...Ch. 3 - Consider the processes shown below. In the...Ch. 3 - Two moles of helium gas axe placed in a...Ch. 3 - An amount of n moles of a monatomic ideal gas in a...Ch. 3 - The temperature of an ideal monatomic gas rises by...Ch. 3 - For a temperature increase of 10 at constant...Ch. 3 - If the gases of the preceding problem are...Ch. 3 - Consider 0.40 mol of dilute carbon dioxide at a...Ch. 3 - When 400 J of heat are slowly added to 10 mol of...Ch. 3 - One of a dilute diatomic gas occupying a volume of...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - An ideal gas has a pressure of 0.50 atm and a...Ch. 3 - Pressure and volume measurements of a dilute gas...Ch. 3 - An ideal monatomic gas at 300 K expands...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - Compare the charge in internal energy of an ideal...Ch. 3 - The temperature of n moles of an ideal gas changes...Ch. 3 - A dilute gas expands quasi-statically to three...Ch. 3 - (a) An ideal gas expands adiabatically from a...Ch. 3 - On an adiabatic process of an ideal gas pressure,...Ch. 3 - Two moles of a monatomic ideal gas such as helium...Ch. 3 - Consider the process shown below. During steps AB...Ch. 3 - A car tile contains 0.0380 m3 of air at a pressure...Ch. 3 - A helium-filled toy balloon has a gauge pressure...Ch. 3 - Steam to drive an old-fashioned steam locomotive...Ch. 3 - A hand-driven tire pump has a piston with a...Ch. 3 - Calculate the net work output of a heat engine...Ch. 3 - What is the net work output of a heat engine that...Ch. 3 - Five moles of a monatomic ideal gas in a cylinder...Ch. 3 - Four moles of a monatomic ideal gas in a cylinder...Ch. 3 - Helium gas is cooled from 20 to 10 by expanding...Ch. 3 - In an adiabatic process, oxygen gas in a container...Ch. 3 - A cylinder containing three moles of a monatomic...Ch. 3 - A cylinder containing three moles of nitrogen gas...Ch. 3 - Two moles of a monatomic ideal gas such as oxygen...Ch. 3 - An insulated vessel contains 1.5 moles of argon at...Ch. 3 - One mole of an ideal monatomic gas occupies a...Ch. 3 - One mole of an ideal gas is initially in a chamber...Ch. 3 - A bullet of mass 10 g is traveling horizontally at...Ch. 3 - The insulated cylinder shown below is closed at...Ch. 3 - In a diesel engine, the fuel is ignited without a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which clade does not include humans? (A)synapsids (B)lobe-fins (C) diapsids (D) osteichthyans
Campbell Biology (11th Edition)
17.1 Reciprocal crosses of experimental animals or plants sometimes give different results in the. What are two...
Genetic Analysis: An Integrated Approach (3rd Edition)
Fill in the blanks: The nose is to the mouth. The ankle is to the knee. The ring finger is to the inde...
Human Anatomy & Physiology (2nd Edition)
Heat lamps are commonly used to maintain foods at about 50C for as long as 12 hours in cafeteria serving lines....
Microbiology: An Introduction
The following results were obtained from a broth dilution test for microbial susceptibility. Antibiotic Concent...
Microbiology: An Introduction
Which coastal area experiences the smallest tidal range? ____________
Applications and Investigations in Earth Science (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The stress-strain diagram for a steel alloy is given in fig. 3. Determine the modulus of elasticity (E). σ (ksi) 40 30 20 10 0 0 0.0005 0.001 0.0015 0.002 0.0025 0.0030.0035 Earrow_forwardA Van de Graff generator, if the metal sphere on the Van de Graff has a charge of 0.14 Coulombs and the person has a mass of 62 kg, how much excess charge would the person need in order to levitate at a distance 25 cm from the center of the charged metal sphere? Assume you can treat both the person and the metal sphere as point charges a distance 25 cm from each other using Coulomb's Law to calculate the electrical force. Give your answer as the number of Coulombsarrow_forwardPlease help me answer the following question. I am having trouble understanding the directions of the things the question is asking for. Please include a detailed explanation and possibly drawings of the directions of Bsource, Binduced, and Iinduced.arrow_forward
- 43. A mass må undergoes circular motion of radius R on a hori- zontal frictionless table, con- nected by a massless string through a hole in the table to a second mass m² (Fig. 5.33). If m₂ is stationary, find expres- sions for (a) the string tension and (b) the period of the circu- lar motion. m2 R m₁ FIGURE 5.33 Problem 43arrow_forwardCH 70. A block is projected up an incline at angle 0. It returns to its initial position with half its initial speed. Show that the coefficient of ki- netic friction is μk = tano.arrow_forwardPassage Problems A spiral is an ice-skating position in which the skater glides on one foot with the other foot held above hip level. It's a required element in women's singles figure-skating competition and is related to the arabesque performed in ballet. Figure 5.40 shows Canadian skater Kaetlyn Osmond executing a spiral during her medal-winning perfor- mance at the 2018 Winter Olympics in Gangneung, South Korea. 77. From the photo, you can conclude that the skater is a. executing a turn to her left. b. executing a turn to her right. c. moving in a straight line out of the page. 78. The net force on the skater a. points to her left. b. points to her right. c. is zero. 79. If the skater were to execute the same maneuver but at higher speed, the tilt evident in the photo would be a. less. b. greater. c. unchanged. FIGURE 5.40 Passage Problems 77-80 80. The tilt angle 0 that the skater's body makes with the vertical is given ap- proximately by 0 = tan¯¹(0.5). From this you can conclude…arrow_forward
- Frictionless surfarrow_forward71. A 2.1-kg mass is connected to a spring with spring constant 72 k = 150 N/m and unstretched length 18 cm. The two are mounted on a frictionless air table, with the free end of the spring attached to a frictionless pivot. The mass is set into circular mo- tion at 1.4 m/s. Find the radius of its path. cor moving at 77 km/h negotiat CH —what's the minimum icient of frictioarrow_forward12. Two forces act on a 3.1-kg mass that undergoes acceleration = 0.91 0.27 m/s². If one force is -1.2î – 2.5ĵ N, what's the other?arrow_forward
- 36. Example 5.7: You whirl a bucket of water around in a vertical circle of radius 1.22 m. What minimum speed at the top of the circle will keep the water in the bucket?arrow_forwardPassage Problems Laptop computers are equipped with accelerometers that sense when the device is dropped and then put the hard drive into a protective mode. Your computer geek friend has written a program that reads the accel- erometer and calculates the laptop's apparent weight. You're amusing yourself with this program on a long plane flight. Your laptop weighs just 5 pounds, and for a long time that's what the program reports. But then the "Fasten Seatbelt" light comes on as the plane encounters turbu- lence. Figure 4.27 shows the readings for the laptop's apparent weight over a 12-second interval that includes the start of the turbulence. 76. At the first sign of turbulence, the plane's acceleration a. is upward. b. is downward. c. is impossible to tell from the graph. 77. The plane's vertical ac- celeration has its greatest magnitude a. during interval B. b. during interval C. c. during interval D. 78. During interval C, you can conclude for certain that the plane is Apparent…arrow_forwardIf the metal sphere on the Van de Graff has a charge of 0.14 Coulombs and the person has a mass of 62 kg, how much excess charge would the person need in order to levitate at a distance 25 cm from the center of the charged metal sphere? Assume you can treat both the person and the metal sphere as point charges a distance 25 cm from each otherarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY