
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
thumb_up100%
Chapter 3, Problem 42P
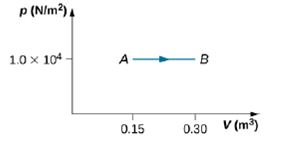
During the isobaric expansion from A to B represented below, 130 J of heat are removed from the gas. What is the change in its internal energy?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Discuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.
Explain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.
3. An Atwood machine consists of two masses, mA
and m B, which are connected by an inelastic cord
of negligible mass that passes over a pulley. If the
pulley has radius RO and
moment of inertia I about its axle, determine the
acceleration of the masses
mA and m B, and compare to the situation where the
moment of inertia of the
pulley is ignored. Ignore friction at the axle O. Use
angular momentum and torque in this solution
Chapter 3 Solutions
University Physics Volume 2
Ch. 3 - The paths ABC, AC, and ADC represent three...Ch. 3 - Check Your Understanding The quantities below...Ch. 3 - Check Your Understanding Why was it necessary to...Ch. 3 - Check Your Understanding When 1.00 g of ammonia...Ch. 3 - Consider these scenarios and state whether work is...Ch. 3 - Is it possible to determine whether a change in...Ch. 3 - When a liquid is vaporized, its change in internal...Ch. 3 - Why does a bicycle pump feel warm as you inflate...Ch. 3 - Is it possible for the temperature of a system to...Ch. 3 - What does the first law of thermodynamics tell us...
Ch. 3 - Does adding heat to a system always increase its...Ch. 3 - A great deal of effort, time, and money has been...Ch. 3 - When a gas expands isothermally, it does work....Ch. 3 - If the pressure and volume of a system are given,...Ch. 3 - It is unlikely that a process can be isothermal...Ch. 3 - How can an object transfer heat if the object does...Ch. 3 - Most materials expand when heated. One notable...Ch. 3 - Why are there two specific heats for gases Cp and...Ch. 3 - Is it possible for to be smaller than unity? `Ch. 3 - Would you expect to be larger for a gas or a...Ch. 3 - There is no change in the internal of an ideal gas...Ch. 3 - Does a gas do any work when it expands...Ch. 3 - A gas follows on an isothermal curve, where p is...Ch. 3 - A mole of gas has isobaric expansion coefficient...Ch. 3 - Find the equation of state of a solid that has an...Ch. 3 - A gas at a pressure of 2.00 atm undergoes a...Ch. 3 - It takes 500 J of work to compress...Ch. 3 - It is found that, when a dilute gas expands...Ch. 3 - In a quasi-static isobaric expansion. 500 J of...Ch. 3 - When a gas undergoes a quasi-static isobaric...Ch. 3 - An ideal gas expands quasi-statically and...Ch. 3 - As shown below, calculate the work done by the gas...Ch. 3 - (a) Calculate the work done by the gas along the...Ch. 3 - An ideal gas expands quasi-statically to three...Ch. 3 - A dilute gas at a pressure of 2.0 atm and a volume...Ch. 3 - What is the average mechanical energy of the atoms...Ch. 3 - What is the internal energy of 6.00 mol of an...Ch. 3 - Calculate the internal energy of 15 mg of helium...Ch. 3 - Two monatomic ideal gases A and B are at the same...Ch. 3 - The van der Waals coefficients for oxygen are...Ch. 3 - Find the work done in the quasi-static processes...Ch. 3 - When a dilute gas expands quasi-statically from...Ch. 3 - In a quasi-static isobaric expansion, 500 J of...Ch. 3 - An ideal gas quasi-statically and isothermally...Ch. 3 - As shown below, if the heat absorbed by the gas...Ch. 3 - During the isobaric expansion from A to B...Ch. 3 - (a) What is the change in internal energy for the...Ch. 3 - When a gas expands along path AC shown below, it...Ch. 3 - When a gas expands along AB (see below), it does...Ch. 3 - A dilute gas is stored in the left chamber of a...Ch. 3 - Ideal gases A and B are stored in the left and...Ch. 3 - An ideal monatomic gas at a pressure of 2.0105N/m2...Ch. 3 - Consider the process for steam in a cylinder shown...Ch. 3 - The state of 30 moles of steam in a cylinder is...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - A metallic container of fixed volume of 2.5103 m3...Ch. 3 - A gas in a cylindrical closed container is...Ch. 3 - Two moles of a monatomic ideal gas at (5 MPa, 5 L)...Ch. 3 - Consider a transformation from point A to B in a...Ch. 3 - Consider a cylinder with a movable piston...Ch. 3 - An ideal gas expands isothermally along AB and...Ch. 3 - Consider the processes shown below. In the...Ch. 3 - Two moles of helium gas axe placed in a...Ch. 3 - An amount of n moles of a monatomic ideal gas in a...Ch. 3 - The temperature of an ideal monatomic gas rises by...Ch. 3 - For a temperature increase of 10 at constant...Ch. 3 - If the gases of the preceding problem are...Ch. 3 - Consider 0.40 mol of dilute carbon dioxide at a...Ch. 3 - When 400 J of heat are slowly added to 10 mol of...Ch. 3 - One of a dilute diatomic gas occupying a volume of...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - An ideal gas has a pressure of 0.50 atm and a...Ch. 3 - Pressure and volume measurements of a dilute gas...Ch. 3 - An ideal monatomic gas at 300 K expands...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - Compare the charge in internal energy of an ideal...Ch. 3 - The temperature of n moles of an ideal gas changes...Ch. 3 - A dilute gas expands quasi-statically to three...Ch. 3 - (a) An ideal gas expands adiabatically from a...Ch. 3 - On an adiabatic process of an ideal gas pressure,...Ch. 3 - Two moles of a monatomic ideal gas such as helium...Ch. 3 - Consider the process shown below. During steps AB...Ch. 3 - A car tile contains 0.0380 m3 of air at a pressure...Ch. 3 - A helium-filled toy balloon has a gauge pressure...Ch. 3 - Steam to drive an old-fashioned steam locomotive...Ch. 3 - A hand-driven tire pump has a piston with a...Ch. 3 - Calculate the net work output of a heat engine...Ch. 3 - What is the net work output of a heat engine that...Ch. 3 - Five moles of a monatomic ideal gas in a cylinder...Ch. 3 - Four moles of a monatomic ideal gas in a cylinder...Ch. 3 - Helium gas is cooled from 20 to 10 by expanding...Ch. 3 - In an adiabatic process, oxygen gas in a container...Ch. 3 - A cylinder containing three moles of a monatomic...Ch. 3 - A cylinder containing three moles of nitrogen gas...Ch. 3 - Two moles of a monatomic ideal gas such as oxygen...Ch. 3 - An insulated vessel contains 1.5 moles of argon at...Ch. 3 - One mole of an ideal monatomic gas occupies a...Ch. 3 - One mole of an ideal gas is initially in a chamber...Ch. 3 - A bullet of mass 10 g is traveling horizontally at...Ch. 3 - The insulated cylinder shown below is closed at...Ch. 3 - In a diesel engine, the fuel is ignited without a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
33. Consider the reaction:
The tabulated data were collected for the concentration of C4H8 as a function...
Chemistry: Structure and Properties (2nd Edition)
The reactions between bleach and ammonia in solution are represented. The difference between given two reaction...
Living By Chemistry: First Edition Textbook
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
2. Whether an allele is dominant or recessive depends on
a. how common the allele is, relative to other alleles...
Campbell Biology: Concepts & Connections (9th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forward
- Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forwardCalculate the center of mass of the hollow cone shown below. Clearly specify the origin and the coordinate system you are using. Z r Y h Xarrow_forward12. If all three collisions in the figure below are totally inelastic, which will cause more damage? (think about which collision has a larger amount of kinetic energy dissipated/lost to the environment? I m II III A. I B. II C. III m m v brick wall ע ע 0.5v 2v 0.5m D. I and II E. II and III F. I and III G. I, II and III (all of them) 2marrow_forward
- 11. If all three collisions in the figure below are totally inelastic, which brings the car of mass (m) on the left to a halt? I m II III m m ע ע ע brick wall 0.5v 2m 2v 0.5m A. I B. II C. III D. I and II E. II and III F. I and III G. I, II and III (all of them)arrow_forwardHow can you tell which vowel is being produced here ( “ee,” “ah,” or “oo”)? Also, how would you be able to tell for the other vowels?arrow_forwardYou want to fabricate a soft microfluidic chip like the one below. How would you go about fabricating this chip knowing that you are targeting a channel with a square cross-sectional profile of 200 μm by 200 μm. What materials and steps would you use and why? Disregard the process to form the inlet and outlet. Square Cross Sectionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY