
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 49P
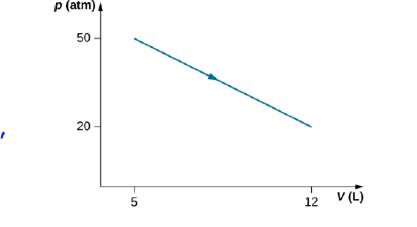
Consider the process for steam in a cylinder shown below. Suppose the change in the internal energy in this process is 30 kJ. Find the heat entering the system.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
!
Required information
The radius of the Moon is 1.737 Mm and the distance between Earth and the Moon is 384.5 Mm.
The intensity of the moonlight incident on her eye is 0.0220 W/m². What is the intensity incident on her retina if the
diameter of her pupil is 6.54 mm and the diameter of her eye is 1.94 cm?
W/m²
Required information
An object is placed 20.0 cm from a converging lens with focal length 15.0 cm (see the figure, not drawn to scale). A
concave mirror with focal length 10.0 cm is located 76.5 cm to the right of the lens. Light goes through the lens, reflects
from the mirror, and passes through the lens again, forming a final image.
Converging
lens
Object
Concave
mirror
15.0 cm
-20.0 cm-
10.0 cm
d cm
d = 76.5.
What is the location of the final image?
cm to the left of the lens
!
Required information
A man requires reading glasses with +2.15-D refractive power to read a book held 40.0 cm away with a relaxed eye.
Assume the glasses are 1.90 cm from his eyes.
His uncorrected near point is 1.00 m. If one of the lenses is the one for distance vision, what should the refractive power of the other
lens (for close-up vision) in his bifocals be to give him clear vision from 25.0 cm to infinity?
2.98 D
Chapter 3 Solutions
University Physics Volume 2
Ch. 3 - The paths ABC, AC, and ADC represent three...Ch. 3 - Check Your Understanding The quantities below...Ch. 3 - Check Your Understanding Why was it necessary to...Ch. 3 - Check Your Understanding When 1.00 g of ammonia...Ch. 3 - Consider these scenarios and state whether work is...Ch. 3 - Is it possible to determine whether a change in...Ch. 3 - When a liquid is vaporized, its change in internal...Ch. 3 - Why does a bicycle pump feel warm as you inflate...Ch. 3 - Is it possible for the temperature of a system to...Ch. 3 - What does the first law of thermodynamics tell us...
Ch. 3 - Does adding heat to a system always increase its...Ch. 3 - A great deal of effort, time, and money has been...Ch. 3 - When a gas expands isothermally, it does work....Ch. 3 - If the pressure and volume of a system are given,...Ch. 3 - It is unlikely that a process can be isothermal...Ch. 3 - How can an object transfer heat if the object does...Ch. 3 - Most materials expand when heated. One notable...Ch. 3 - Why are there two specific heats for gases Cp and...Ch. 3 - Is it possible for to be smaller than unity? `Ch. 3 - Would you expect to be larger for a gas or a...Ch. 3 - There is no change in the internal of an ideal gas...Ch. 3 - Does a gas do any work when it expands...Ch. 3 - A gas follows on an isothermal curve, where p is...Ch. 3 - A mole of gas has isobaric expansion coefficient...Ch. 3 - Find the equation of state of a solid that has an...Ch. 3 - A gas at a pressure of 2.00 atm undergoes a...Ch. 3 - It takes 500 J of work to compress...Ch. 3 - It is found that, when a dilute gas expands...Ch. 3 - In a quasi-static isobaric expansion. 500 J of...Ch. 3 - When a gas undergoes a quasi-static isobaric...Ch. 3 - An ideal gas expands quasi-statically and...Ch. 3 - As shown below, calculate the work done by the gas...Ch. 3 - (a) Calculate the work done by the gas along the...Ch. 3 - An ideal gas expands quasi-statically to three...Ch. 3 - A dilute gas at a pressure of 2.0 atm and a volume...Ch. 3 - What is the average mechanical energy of the atoms...Ch. 3 - What is the internal energy of 6.00 mol of an...Ch. 3 - Calculate the internal energy of 15 mg of helium...Ch. 3 - Two monatomic ideal gases A and B are at the same...Ch. 3 - The van der Waals coefficients for oxygen are...Ch. 3 - Find the work done in the quasi-static processes...Ch. 3 - When a dilute gas expands quasi-statically from...Ch. 3 - In a quasi-static isobaric expansion, 500 J of...Ch. 3 - An ideal gas quasi-statically and isothermally...Ch. 3 - As shown below, if the heat absorbed by the gas...Ch. 3 - During the isobaric expansion from A to B...Ch. 3 - (a) What is the change in internal energy for the...Ch. 3 - When a gas expands along path AC shown below, it...Ch. 3 - When a gas expands along AB (see below), it does...Ch. 3 - A dilute gas is stored in the left chamber of a...Ch. 3 - Ideal gases A and B are stored in the left and...Ch. 3 - An ideal monatomic gas at a pressure of 2.0105N/m2...Ch. 3 - Consider the process for steam in a cylinder shown...Ch. 3 - The state of 30 moles of steam in a cylinder is...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - A metallic container of fixed volume of 2.5103 m3...Ch. 3 - A gas in a cylindrical closed container is...Ch. 3 - Two moles of a monatomic ideal gas at (5 MPa, 5 L)...Ch. 3 - Consider a transformation from point A to B in a...Ch. 3 - Consider a cylinder with a movable piston...Ch. 3 - An ideal gas expands isothermally along AB and...Ch. 3 - Consider the processes shown below. In the...Ch. 3 - Two moles of helium gas axe placed in a...Ch. 3 - An amount of n moles of a monatomic ideal gas in a...Ch. 3 - The temperature of an ideal monatomic gas rises by...Ch. 3 - For a temperature increase of 10 at constant...Ch. 3 - If the gases of the preceding problem are...Ch. 3 - Consider 0.40 mol of dilute carbon dioxide at a...Ch. 3 - When 400 J of heat are slowly added to 10 mol of...Ch. 3 - One of a dilute diatomic gas occupying a volume of...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - An ideal gas has a pressure of 0.50 atm and a...Ch. 3 - Pressure and volume measurements of a dilute gas...Ch. 3 - An ideal monatomic gas at 300 K expands...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - Compare the charge in internal energy of an ideal...Ch. 3 - The temperature of n moles of an ideal gas changes...Ch. 3 - A dilute gas expands quasi-statically to three...Ch. 3 - (a) An ideal gas expands adiabatically from a...Ch. 3 - On an adiabatic process of an ideal gas pressure,...Ch. 3 - Two moles of a monatomic ideal gas such as helium...Ch. 3 - Consider the process shown below. During steps AB...Ch. 3 - A car tile contains 0.0380 m3 of air at a pressure...Ch. 3 - A helium-filled toy balloon has a gauge pressure...Ch. 3 - Steam to drive an old-fashioned steam locomotive...Ch. 3 - A hand-driven tire pump has a piston with a...Ch. 3 - Calculate the net work output of a heat engine...Ch. 3 - What is the net work output of a heat engine that...Ch. 3 - Five moles of a monatomic ideal gas in a cylinder...Ch. 3 - Four moles of a monatomic ideal gas in a cylinder...Ch. 3 - Helium gas is cooled from 20 to 10 by expanding...Ch. 3 - In an adiabatic process, oxygen gas in a container...Ch. 3 - A cylinder containing three moles of a monatomic...Ch. 3 - A cylinder containing three moles of nitrogen gas...Ch. 3 - Two moles of a monatomic ideal gas such as oxygen...Ch. 3 - An insulated vessel contains 1.5 moles of argon at...Ch. 3 - One mole of an ideal monatomic gas occupies a...Ch. 3 - One mole of an ideal gas is initially in a chamber...Ch. 3 - A bullet of mass 10 g is traveling horizontally at...Ch. 3 - The insulated cylinder shown below is closed at...Ch. 3 - In a diesel engine, the fuel is ignited without a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Classify each molecule as polar nonpolar. a. CS2 b. SO2 c. CH4 d. CH3CI
Introductory Chemistry (6th Edition)
MAKE CONNECTIONS In Concept 20.2, you learned about genome-wide association studies. Explain how these studies...
Campbell Biology (11th Edition)
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- ! Required information Assume that the magnifier is held close to the eye. Use the standard near point of 25.0 cm to find the angular magnification. An insect that is 4.10 mm long is placed 10.3 cm from a simple magnifier with a focal length of 13.0 cm. What is the angular magnification?arrow_forward2arrow_forward3arrow_forward
- Imagine you are out for a stroll on a sunny day when you encounter a lake. Unpolarized light from the sun is reflected off the lake into your eyes. However, you notice when you put on your vertically polarized sunglasses, the light reflected off the lake no longer reaches your eyes. What is the angle between the unpolarized light and the surface of the water, in degrees, measured from the horizontal? You may assume the index of refraction of air is nair=1 and the index of refraction of water is nwater=1.33 . Round your answer to three significant figures. Just enter the number, nothing else.arrow_forwardDeduce what overvoltage is like in reversible electrodes.arrow_forwardpls help on thesearrow_forward
- pls help on thesearrow_forward20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY