
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 45P
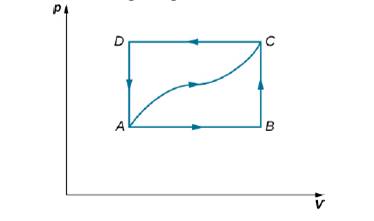
When a gas expands along AB (see below), it does 500 J of work and absorbs 250 J of heat. When the gas expands along AC, it does 700 J of work and absorbs 300 J of heat. (a) How much heat does the gas exchange along BC? (b) When the gas makes the transmission from C to A along CDA, 800 J of work are done on it from C to D. How much heat does it exchange along CDA?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank you
A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).
What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V)
ammeter
I =
Chapter 3 Solutions
University Physics Volume 2
Ch. 3 - The paths ABC, AC, and ADC represent three...Ch. 3 - Check Your Understanding The quantities below...Ch. 3 - Check Your Understanding Why was it necessary to...Ch. 3 - Check Your Understanding When 1.00 g of ammonia...Ch. 3 - Consider these scenarios and state whether work is...Ch. 3 - Is it possible to determine whether a change in...Ch. 3 - When a liquid is vaporized, its change in internal...Ch. 3 - Why does a bicycle pump feel warm as you inflate...Ch. 3 - Is it possible for the temperature of a system to...Ch. 3 - What does the first law of thermodynamics tell us...
Ch. 3 - Does adding heat to a system always increase its...Ch. 3 - A great deal of effort, time, and money has been...Ch. 3 - When a gas expands isothermally, it does work....Ch. 3 - If the pressure and volume of a system are given,...Ch. 3 - It is unlikely that a process can be isothermal...Ch. 3 - How can an object transfer heat if the object does...Ch. 3 - Most materials expand when heated. One notable...Ch. 3 - Why are there two specific heats for gases Cp and...Ch. 3 - Is it possible for to be smaller than unity? `Ch. 3 - Would you expect to be larger for a gas or a...Ch. 3 - There is no change in the internal of an ideal gas...Ch. 3 - Does a gas do any work when it expands...Ch. 3 - A gas follows on an isothermal curve, where p is...Ch. 3 - A mole of gas has isobaric expansion coefficient...Ch. 3 - Find the equation of state of a solid that has an...Ch. 3 - A gas at a pressure of 2.00 atm undergoes a...Ch. 3 - It takes 500 J of work to compress...Ch. 3 - It is found that, when a dilute gas expands...Ch. 3 - In a quasi-static isobaric expansion. 500 J of...Ch. 3 - When a gas undergoes a quasi-static isobaric...Ch. 3 - An ideal gas expands quasi-statically and...Ch. 3 - As shown below, calculate the work done by the gas...Ch. 3 - (a) Calculate the work done by the gas along the...Ch. 3 - An ideal gas expands quasi-statically to three...Ch. 3 - A dilute gas at a pressure of 2.0 atm and a volume...Ch. 3 - What is the average mechanical energy of the atoms...Ch. 3 - What is the internal energy of 6.00 mol of an...Ch. 3 - Calculate the internal energy of 15 mg of helium...Ch. 3 - Two monatomic ideal gases A and B are at the same...Ch. 3 - The van der Waals coefficients for oxygen are...Ch. 3 - Find the work done in the quasi-static processes...Ch. 3 - When a dilute gas expands quasi-statically from...Ch. 3 - In a quasi-static isobaric expansion, 500 J of...Ch. 3 - An ideal gas quasi-statically and isothermally...Ch. 3 - As shown below, if the heat absorbed by the gas...Ch. 3 - During the isobaric expansion from A to B...Ch. 3 - (a) What is the change in internal energy for the...Ch. 3 - When a gas expands along path AC shown below, it...Ch. 3 - When a gas expands along AB (see below), it does...Ch. 3 - A dilute gas is stored in the left chamber of a...Ch. 3 - Ideal gases A and B are stored in the left and...Ch. 3 - An ideal monatomic gas at a pressure of 2.0105N/m2...Ch. 3 - Consider the process for steam in a cylinder shown...Ch. 3 - The state of 30 moles of steam in a cylinder is...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - A metallic container of fixed volume of 2.5103 m3...Ch. 3 - A gas in a cylindrical closed container is...Ch. 3 - Two moles of a monatomic ideal gas at (5 MPa, 5 L)...Ch. 3 - Consider a transformation from point A to B in a...Ch. 3 - Consider a cylinder with a movable piston...Ch. 3 - An ideal gas expands isothermally along AB and...Ch. 3 - Consider the processes shown below. In the...Ch. 3 - Two moles of helium gas axe placed in a...Ch. 3 - An amount of n moles of a monatomic ideal gas in a...Ch. 3 - The temperature of an ideal monatomic gas rises by...Ch. 3 - For a temperature increase of 10 at constant...Ch. 3 - If the gases of the preceding problem are...Ch. 3 - Consider 0.40 mol of dilute carbon dioxide at a...Ch. 3 - When 400 J of heat are slowly added to 10 mol of...Ch. 3 - One of a dilute diatomic gas occupying a volume of...Ch. 3 - A monatomic ideal gas undergoes a quasi-static...Ch. 3 - An ideal gas has a pressure of 0.50 atm and a...Ch. 3 - Pressure and volume measurements of a dilute gas...Ch. 3 - An ideal monatomic gas at 300 K expands...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - An ideal diatomic gas at 80 K is slowly compressed...Ch. 3 - Compare the charge in internal energy of an ideal...Ch. 3 - The temperature of n moles of an ideal gas changes...Ch. 3 - A dilute gas expands quasi-statically to three...Ch. 3 - (a) An ideal gas expands adiabatically from a...Ch. 3 - On an adiabatic process of an ideal gas pressure,...Ch. 3 - Two moles of a monatomic ideal gas such as helium...Ch. 3 - Consider the process shown below. During steps AB...Ch. 3 - A car tile contains 0.0380 m3 of air at a pressure...Ch. 3 - A helium-filled toy balloon has a gauge pressure...Ch. 3 - Steam to drive an old-fashioned steam locomotive...Ch. 3 - A hand-driven tire pump has a piston with a...Ch. 3 - Calculate the net work output of a heat engine...Ch. 3 - What is the net work output of a heat engine that...Ch. 3 - Five moles of a monatomic ideal gas in a cylinder...Ch. 3 - Four moles of a monatomic ideal gas in a cylinder...Ch. 3 - Helium gas is cooled from 20 to 10 by expanding...Ch. 3 - In an adiabatic process, oxygen gas in a container...Ch. 3 - A cylinder containing three moles of a monatomic...Ch. 3 - A cylinder containing three moles of nitrogen gas...Ch. 3 - Two moles of a monatomic ideal gas such as oxygen...Ch. 3 - An insulated vessel contains 1.5 moles of argon at...Ch. 3 - One mole of an ideal monatomic gas occupies a...Ch. 3 - One mole of an ideal gas is initially in a chamber...Ch. 3 - A bullet of mass 10 g is traveling horizontally at...Ch. 3 - The insulated cylinder shown below is closed at...Ch. 3 - In a diesel engine, the fuel is ignited without a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Sketch the following spectra that would be obtained for 2-chloroethanol: a. The 1H NMR spectrum for an anhydrou...
Organic Chemistry (8th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology with Physiology (5th Edition)
2. List the subdivisions of the thoracic and abdominopelvic cavities.
Human Anatomy & Physiology (2nd Edition)
Which coastal area experiences the smallest tidal range? ____________
Applications and Investigations in Earth Science (9th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
During exponential growth, a population always (A) has a constant per capita population growth rate. (B) quickl...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- simple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forward
- A long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forwardDiscuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forward
- Explain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forwardA 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forward
- For each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forwardTwo complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY