
Concept explainers
(a)
Interpretation: To determine whether ornithine is associated with (1) transamination, (2) oxidative deamination, or (3) the urea cycle.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(a)
Answer to Problem 26.74EP
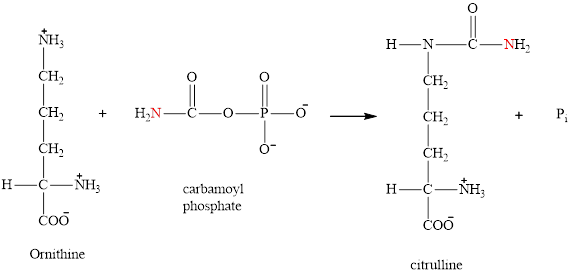
Ornithine is associated with the urea cycle.
Explanation of Solution
Ornithine is a nonstandard amino acid and accepts the entering carbamoyl phosphate group at the start of each urea cycle. It is encountered in the first step of the urea cycle.
(b)
Interpretation: To determine whether
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(b)
Answer to Problem 26.74EP
The ammonium ion
Explanation of Solution
The ammonium ion is a product of oxidative deamination reaction. A net oxidative deamination reaction is as follows:
It is also associated with the urea cycle. It is fuel for the urea cycle however; it is first converted into carbamoyl phosphate and then enters into the cycle. The net urea cycle is as follows:
(c)
Interpretation: To determine whether
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(c)
Answer to Problem 26.74EP
Explanation of Solution
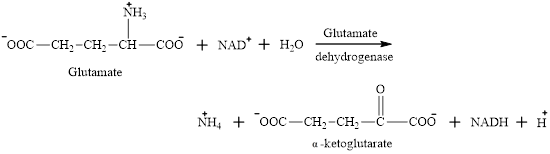
Oxidative deamination reaction of glutamate requires dehydrogenase enzyme. It is an oxidoreductase enzyme and works with either
The oxidative deamination reaction of glutamate amino acid is as follows:
(d)
Interpretation: To determine whether aspartate is associated with (1) transamination, (2) oxidative deamination, or (3) the urea cycle.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A urea cycle is a cyclic biochemical pathway that involves the production of urea using ammonium ions and aspartate molecules as nitrogen sources. The reactants in the formation of carbamoyl phosphate are ammonium ion, water, and carbon dioxide.
(d)
Answer to Problem 26.74EP
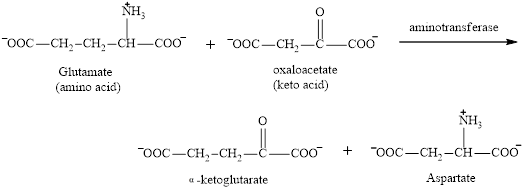
Aspartate is associated with the transamination reaction and urea cycle.
Explanation of Solution
Aspartate is a non-essential amino acid. It could function as a product in the transamination reaction.
The reaction is as follows:
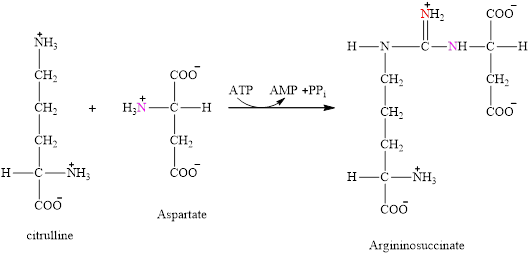
Aspartate is also associated with the urea cycle. It is fuel for the urea cycle. It enters directly in step 2 of the cycle and condenses with citrulline to produce argininosuccinate.
Want to see more full solutions like this?
Chapter 26 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





