
Concept explainers
(a)
Interpretation: To identify whether the statement “cysteine and serine are standard amino acids that contain the element sulfur” concerning sulfur-containing amino acids is true or false.
Concept introduction: Amino acids are the
There are 20 amino acids present in nature. These are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, alanine, asparagines, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
(b)
Interpretation: To identify whether the statement “the molecule
Concept introduction: There are 20 amino acids present in nature. These are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, alanine, asparagines, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine. Only two of the twenty amino acids have a sulfur atom in their structure. The two amino acids are cysteine and methionine. The structure of cysteine is:
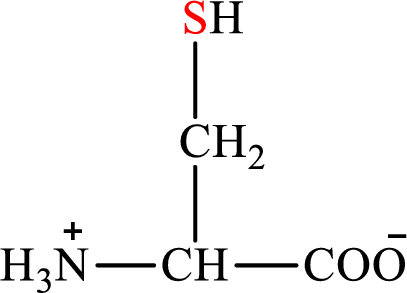
Cysteine gives pyruvate as the degradation product. The degradation of cysteine is a two-step process.
(c)
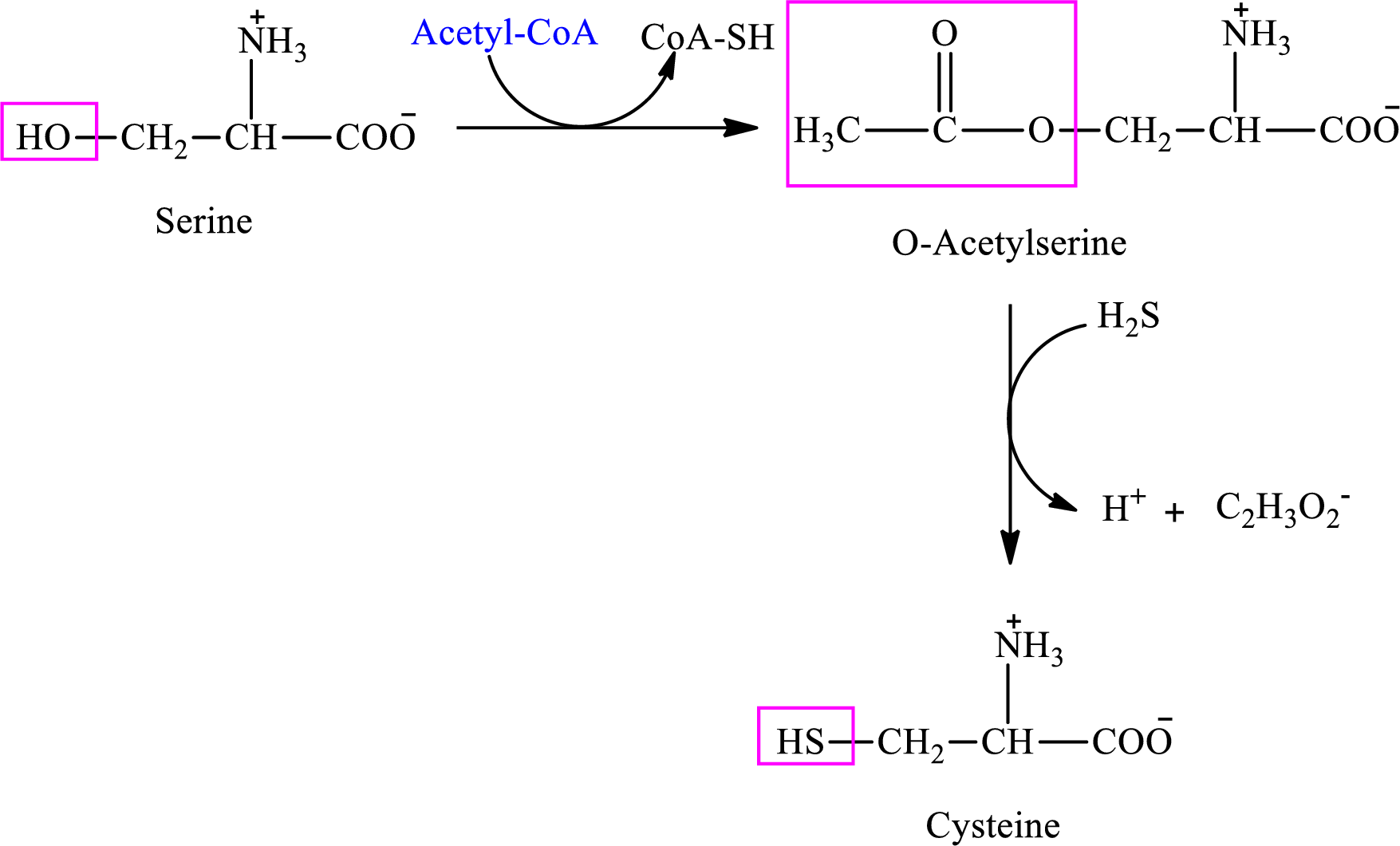
Interpretation: To identify whether the statement “the first step in the synthesis of cysteine from serine is an activation step” concerning sulfur-containing amino acids is true or false.
Concept introduction: There are 20 amino acids present in nature. Only two of the twenty amino acids have a sulfur atom in their structure. The two amino acids are cysteine and methionine. The structure of cysteine is:
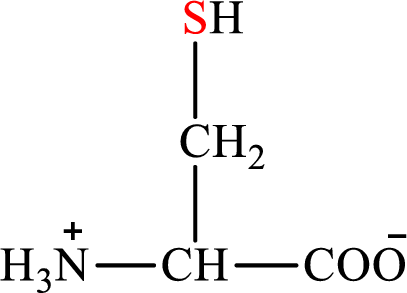
Cysteine is biosynthesized from serine that involves two steps.
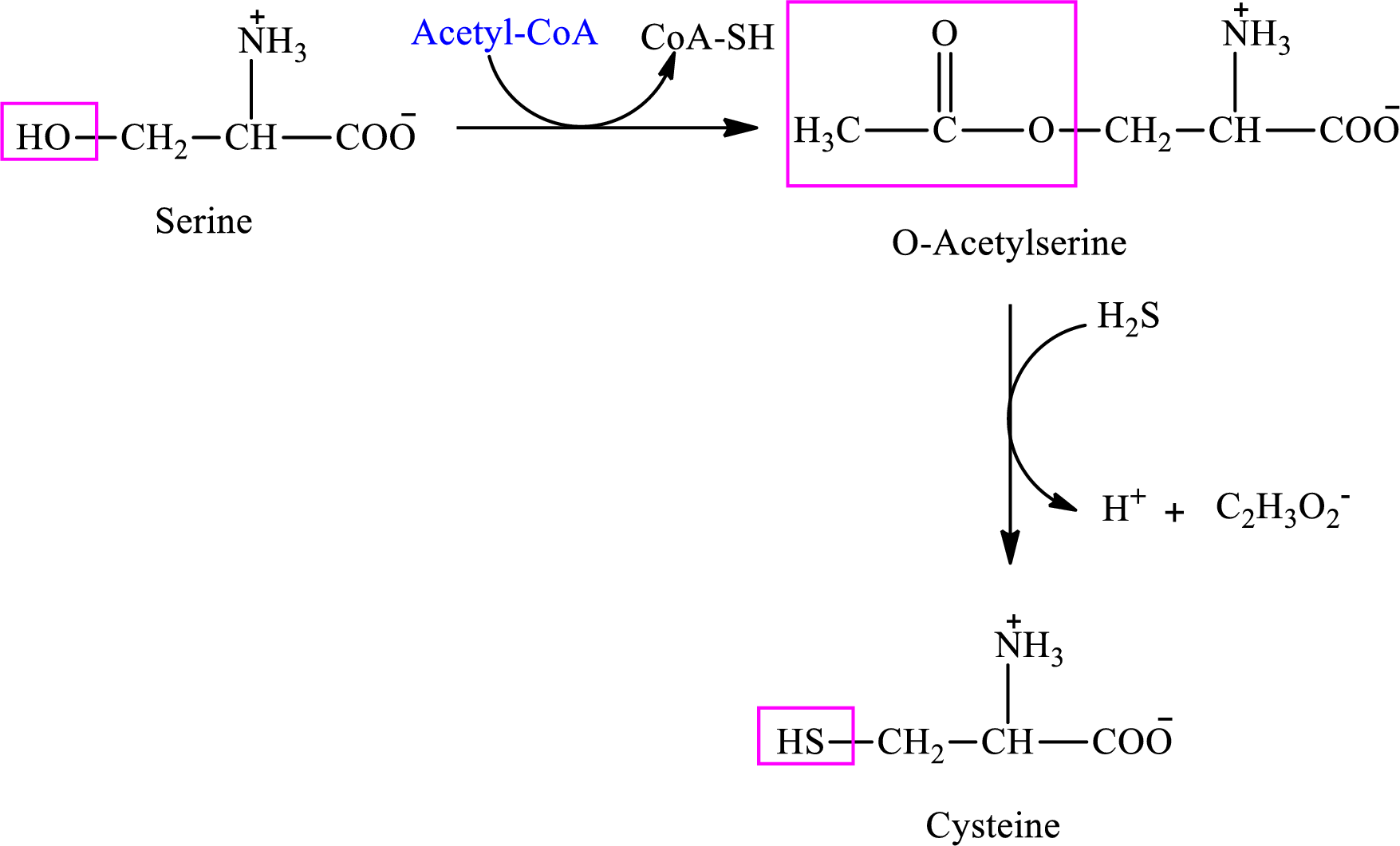
(d)
Interpretation: To identify whether the statement “in the synthesis of cysteine from serine it is
Concept introduction: There are 20 amino acids present in nature. Only two of the twenty amino acids have a sulfur atom in their structure. The two amino acids are cysteine and methionine. The structure of cysteine is:
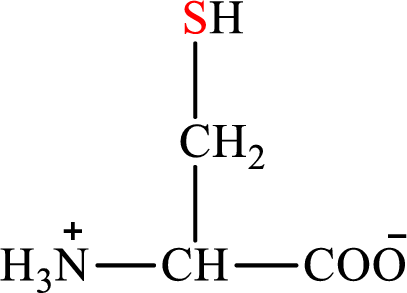
Cysteine is biosynthesized from serine that involves two steps.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction LiNO3arrow_forwardAn unknown weak acid with a concentration of 0.410 M has a pH of 5.600. What is the Ka of the weak acid?arrow_forward(racemic) 19.84 Using your reaction roadmaps as a guide, show how to convert 2-oxepanone and ethanol into 1-cyclopentenecarbaldehyde. You must use 2-oxepanone as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way. & + EtOH H 2-Oxepanone 1-Cyclopentenecarbaldehydearrow_forward
- R₂ R₁ R₁ a R Rg Nu R₂ Rg R₁ R R₁₂ R3 R R Nu enolate forming R₁ R B-Alkylated carbonyl species or amines Cyclic B-Ketoester R₁₁ HOB R R₁B R R₁₂ B-Hydroxy carbonyl R diester R2 R3 R₁ RB OR R₂ 0 aB-Unsaturated carbonyl NaOR Aldol HOR reaction 1) LDA 2) R-X 3) H₂O/H₂O ketone, aldehyde 1) 2°-amine 2) acid chloride 3) H₂O'/H₂O 0 O R₁ R₁ R R₁ R₁₂ Alkylated a-carbon R₁ H.C R₁ H.C Alkylated methyl ketone acetoacetic ester B-Ketoester ester R₁ HO R₂ R B-Dicarbonyl HO Alkylated carboxylic acid malonic ester Write the reagents required to bring about each reaction next to the arrows shown. Next, record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as forma- tion of an important intermediate, as a helpful reminder. You may want to keep track of all reactions that make carbon-carbon bonds, because these help you build large molecules from smaller fragments. This especially applies to the reactions in…arrow_forwardProvide the reasonable steps to achieve the following synthesis.arrow_forwardIdentify which compound is more acidic. Justify your choice.arrow_forward
- Provide the reasonable steps to achieve the following synthesis.arrow_forwardWhen anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





