
Combine the following structures to create a ribonucleotide. Show where water is removed to form an N-glycosidic linkage and where water is removed to form a phosphate ester. Draw the resulting ribonucleotide structure, and name it.
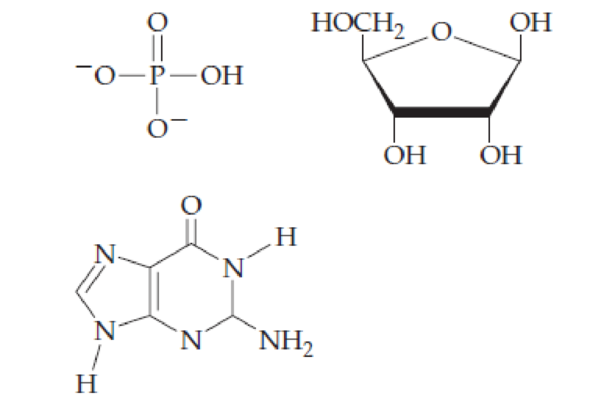
Interpretation:
The ribonucleotide structure has to be predicted and named.
Concept Introduction:
Composition of nucleic acid: Nucleic acid is a polymer of nucleotides. Each nucleotide has three parts: a sugar, a nitrogenous base, and a phosphate group.
Sugar: In both DNA and RNA, sugar portion is found. In DNA, the sugar is D-ribose, where at 2’hydroxyl group is absent and in RNA, the hydroxyl group is present at 2’.
Nitrogenous bases: Five types of nitrogenous bases (has unique one-letter code A, G, T, U, and C) are derived from two parent compounds called purine and pyrimidine. The purine derivatives are Adenine and Guanine are two fused rings. The pyrimidine derivatives are six-membered nitrogen containing ring. Adenine, Guanine, Thymine, and Cytosine are the nitrogenous bases present in DNA. Adenine, Guanine, Cytosine and Uracil are the nitrogenous bases present in RNA.
Nucleotide: (Nucleoside + phosphate)
Nucleotides are the building blocks of nuclei acids; monomers of DNA and RNA polymers. At carbon-5’ of the ribose sugar, a phosphate group is added which is collectively known as nucleotide. Phosphate groups can be added to any of the nucleotide to form diphosphate or triphosphate.
Nucleoside and its naming: The combination of monosaccharide (sugar) and nitrogenous base is known as nucleoside. The nucleoside names are the nitrogenous base name modified with criteria. While naming nucleoside of purine derivatives the suffix ‘-osine’ is included and for pyrimidine derivatives the suffix ‘-idine’ is used. No prefix used for the nucleosides containing ribose and the prefix ‘deoxy-’ is used for deoxyribose.
Naming nucleotide: At the end of the nucleoside, phosphate group is added. For example, 5’-monophosphate means adding one phosphate group at 5’carbon in the sugar ring.
Numbering the atoms in sugar and base rings:
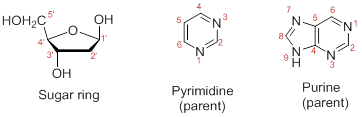
In order to distinguish the atoms in the sugar of a nucleoside and atoms of a base ring, numbers without prime is used for atoms in the base ring and numbers with prime used for the atoms in the sugar ring.
Answer to Problem 26.22UKC
The structure is,
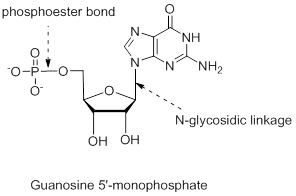
Explanation of Solution
The nucleotide structure is a combination of nucleoside [ribose (presence of –OH group at 2’ carbon atom in the sugar ring) and guanine base (derivative of purine parent)]. The nucleoside name is the nitrogenous name itself; thus, name is Guanine. Looking at the criteria the name of nitrogenous base is modified as follows, the suffix ‘-osine’ is used for the purine derivatives, as here it is Adenine to Guanosine.
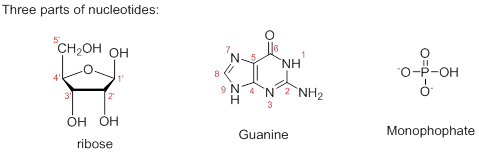
Therefore, the structure of given Guanosine 5’-monophosphate is,
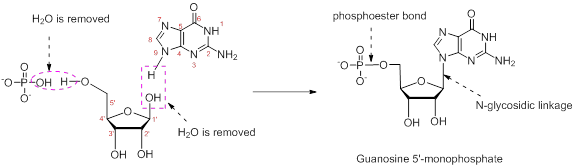
The ribonucleotide structure is predicted and named.
Want to see more full solutions like this?
Chapter 26 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Additional Science Textbook Solutions
Biological Science (6th Edition)
Biology: Life on Earth (11th Edition)
Genetics: From Genes to Genomes
Fundamentals of Anatomy & Physiology (11th Edition)
Chemistry & Chemical Reactivity
Campbell Essential Biology (7th Edition)
- 9. Which one of the compounds below is a major final product of the reaction sequence shown at the right? A) para-bromonitrobenzene C) meta-bromoaniline B) meta-bromonitrobenzene D) para-bromoaniline 1. HNO3, H2SO4 2. Br₂, FeBr3 3. H₂/Ni (3 atm) E) ortho-bromoanilinearrow_forward10. This reaction sequence includes an intramolecular Friedel-Crafts reaction. Which of the compounds below is expected to be the major product? PhCH2CH2CH2COOH 4-phenylbutanoic acid SOCI₂ AICI 3 A B C D Earrow_forward5. Which one is the major organic product obtained from the following reaction sequence? A B C OH i 1. NaBH4 CI 2. H₂O, H+ AICI 3 D OH Earrow_forward
- 1. Which one is the major organic product obtained from the reaction of toluene and cyclopentanol in the presence of H3PO4, as shown here? CH3 CH3 CH3 CH3 CH3 H3PO4 A B с D E OHarrow_forwardAscorbic acid is a diprotic, with ionizations of: pKa1 = 4.10; pKa2 =11.80. You need to make 350 mL of an ascorbate buffer that is pH 5.05, andyou have 1.5 mM stock solutions of :ascorbic acidmonosodium ascorbatedipotassium ascorbateHow much 1.5 mM monosodium ascorbate do you use to make yoursolution? Answer in mL and report your value to three signicant gures.Please type only the number.arrow_forward1. What is the abbreviated form of the name for the molecule below. Punctuate it correctly ( image attached) 2. How much ATP is formed by the complete oxidation of lignocerate? Show stepsarrow_forward
- fill in the blank and circle the active site for each molecule. urgent!arrow_forwardfill in the table and circle the active sitearrow_forwardThe two half reactions for beginning and end of the electron transport chain are given below in standard form. Calculate & for the overall process. Using the Nernst equation (AG° = -n Fo, F= 96.485 kJ/volt mol), calculate AG°. Explain the need for a stepwise process in the electron transport chain. NAD* + H+ + 2 e- = NADH ½ 0г + 2H+ + 2е- = H20 = -0.32v E = +0.82Varrow_forward
- answer the questions and the example steps should be from carbohydrates glycolysis and citric acid cycle. Please put down reactions and structuresarrow_forwardidentify the general type of reaction catalyzed and an example step from glycolisis structure for each of the following enzymes/ co factor Kinase, isomerase, mutase, dehydrogenase, NAD+ , FADarrow_forwardfill in the blanks with the missing structures and give namesarrow_forward
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





