
Biochemistry
9th Edition
ISBN: 9781305961135
Author: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 47RE
Interpretation Introduction
Interpretation:
The noncatalytic step, the number of molecules of 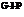 that would form from one molecule of epinephrine, and the biochemical advantage of such a cascade are to be determined.
that would form from one molecule of epinephrine, and the biochemical advantage of such a cascade are to be determined.
Concept introduction:
The chemical messengers that pass information from the source part to the target part are called hormones.
A molecule that acts as a bridge between the hormone and the target part is called the second messenger.
The hormones that principally controls carbohydrate
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The beta-lactamase hydrolyzes the lactam-ring in penicillin. Describe the mechanism
of hydrolysis, insuring to include the involvement of S, D, & K in the reaction sequence. Please help
To map the active site of beta-lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine.
Why doesn't D in this hexapeptide not participate in the hydrolysis of the beta-lactam ring even though S, K, and D are involved in the catalyst?
To map the active site of -lactamase, the enzyme was hydrolyzed with trypsin to yield a hexapeptide (P1) with the following amino acids. Glu, Lys, Leu, Phe, Met, and Ser. Treatment of P1 with phenyl isothiocyanate yielded a PTH derivative of phenylalanine and a peptide (P2). Treatment of P1 with cyanogenbromide gave an acidic tetrapeptide (P3) and a dipeptide (P4).Treatment of P2 with 1-fluoro-2,4-dinitrobenzene, followed by complete hydrolysis, yields N-2,4-dinitrophenyl-Glu. P1, P2, and P3 contain the active site serine.
Using the experimental results described above derive the primary sequence of the active site hexapeptide. Please help!
Chapter 24 Solutions
Biochemistry
Ch. 24 - RECALL What are the two primary molecules that...Ch. 24 - Prob. 2RECh. 24 - RECALL Many components of the glycolytic pathway...Ch. 24 - Prob. 4RECh. 24 - Prob. 5RECh. 24 - REFLECT AND APPLY Why is it somewhat misleading to...Ch. 24 - Prob. 7RECh. 24 - Prob. 8RECh. 24 - Prob. 9RECh. 24 - Prob. 11RE
Ch. 24 - RECALL What do we mean when we say that there is...Ch. 24 - Prob. 13RECh. 24 - Prob. 14RECh. 24 - Prob. 15RECh. 24 - Prob. 16RECh. 24 - BIOCHEMICAL CONNECTIONS People who are both...Ch. 24 - Prob. 18RECh. 24 - Prob. 19RECh. 24 - Prob. 20RECh. 24 - REFLECT AND APPLY Immature rats are fed all the...Ch. 24 - Prob. 22RECh. 24 - Prob. 23RECh. 24 - BIOCHEMICAL CONNECTIONS During colonial times,...Ch. 24 - Prob. 25RECh. 24 - Prob. 26RECh. 24 - Prob. 27RECh. 24 - Prob. 28RECh. 24 - Prob. 29RECh. 24 - Prob. 30RECh. 24 - Prob. 31RECh. 24 - Prob. 32RECh. 24 - Prob. 33RECh. 24 - RECALL The hormone thyroxine is given as an oral...Ch. 24 - Prob. 35RECh. 24 - Prob. 36RECh. 24 - Prob. 37RECh. 24 - Prob. 38RECh. 24 - Prob. 39RECh. 24 - Prob. 40RECh. 24 - Prob. 41RECh. 24 - Prob. 42RECh. 24 - Prob. 43RECh. 24 - Prob. 44RECh. 24 - Prob. 45RECh. 24 - Prob. 46RECh. 24 - Prob. 47RECh. 24 - Prob. 48RECh. 24 - Prob. 49RECh. 24 - Prob. 50RECh. 24 - Prob. 51RECh. 24 - Prob. 52RECh. 24 - Prob. 53RECh. 24 - Prob. 54RECh. 24 - Prob. 55RECh. 24 - Prob. 56RECh. 24 - Prob. 57RECh. 24 - Prob. 58RECh. 24 - Prob. 59RECh. 24 - Prob. 60RECh. 24 - Prob. 61RECh. 24 - Prob. 62RECh. 24 - BIOCHEMICAL CONNECTIONS What is the natural...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward+NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
- Which features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forwardLooking at the figure 30-5 what intermolecular forces are present between the substrate and the enzyme and the substrate and cofactors.arrow_forwardprovide short answers to the followings Urgent!arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvatedehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanismfor this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency inany one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and ischaracterized by cardiac and neurological symptoms. One key diagnostic forthis disease is an increased level of pyruvate and α-ketoglutarate in thebloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardDraw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle - (Draw the Mechanism). How many rounds of Krebs will be required to waste all Carbons of Glutamic Acidas CO2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY