
Concept explainers
(a)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the
The block diagram to represent an overview of glycolysis is as follows:
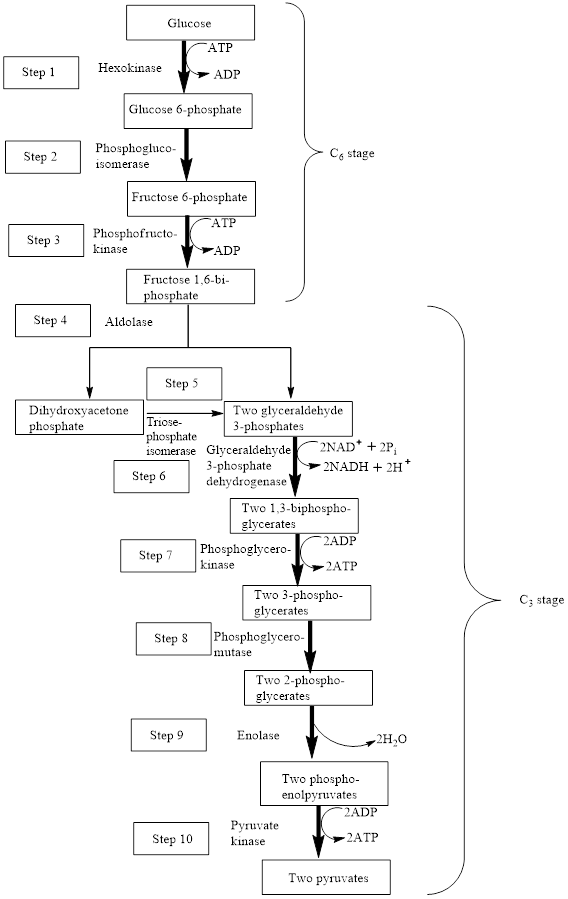
From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
(b)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH reduced coenzymes. The process of glycolysis is a ten-step process in which each step is catalyzed by enzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the cleavage reaction, the carbon-carbon bond is cleaved to form a new bond between carbon and oxygen atom.
(c)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH reduced coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
(d)
Interpretation: To identify the name of the missing substance in the following word equation.
Concept introduction: Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH reduced coenzymes.
The block diagram to represent an overview of glycolysis is as follows:

From the above diagram, it is concluded that in the overall process of glycolysis, two stages are present.
a) Steps 1 to 3 represents a six-carbon stage
b) Steps 4 to 10 represent a three-carbon stage
In the isomerization reaction, a molecule transformed itself to another molecule, having the same number of atoms with a different arrangement.
Enzymes are defined as specialized proteins that act as biological catalysts. Enzymes accelerate the biochemical reactions to produce the substances that are needed for cells for their proper functioning and are very specific to their action.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





