
Concept explainers
(a)
Interpretation: To indicate whether lactate is involved in (1) the pentose phosphate pathway, (2) the Cori cycle, (3) glycolysis, or (4) lactate fermentation.
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Fermentation is defined as the biochemical anaerobic process by which NADH is oxidized to
The structure of lactate is as follows:
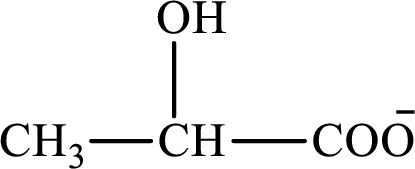
(a)
Answer to Problem 24.105EP
Lactate is associated with (2) the Cori cycle and (4) the lactate fermentation.
Explanation of Solution
An overview of the Cori cycle is as follows:
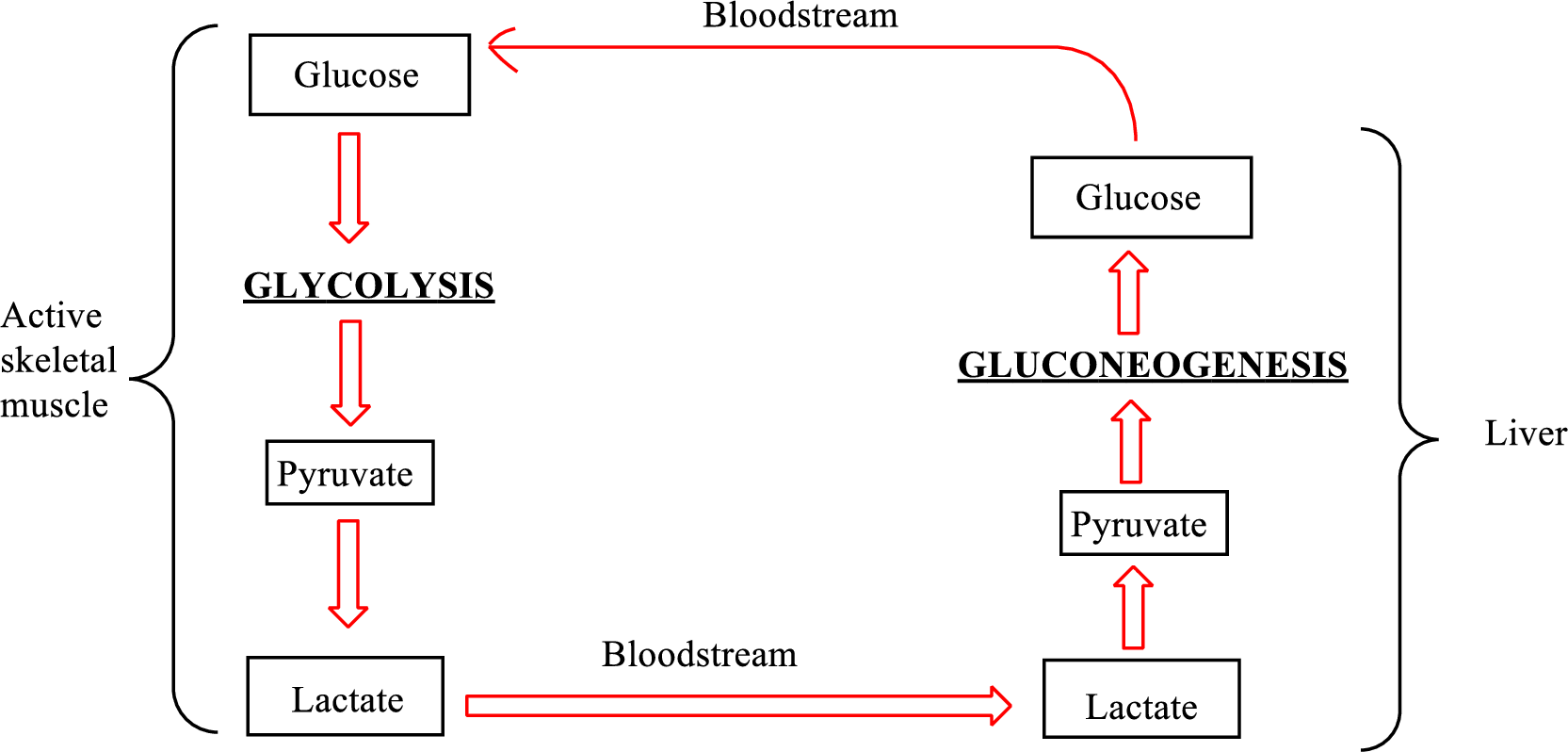
Lactate is converted to pyruvate in the liver. Therefore, lactate is associated with the Cori cycle.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation. The
Therefore, lactate is associated with the Cori cycle and the lactate fermentation.
(b)
Interpretation: To indicate whether
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
Nicotinamide adenine dinucleotide is associated with the
(b)
Answer to Problem 24.105EP
Explanation of Solution
An overview of the Cori cycle is as follows:
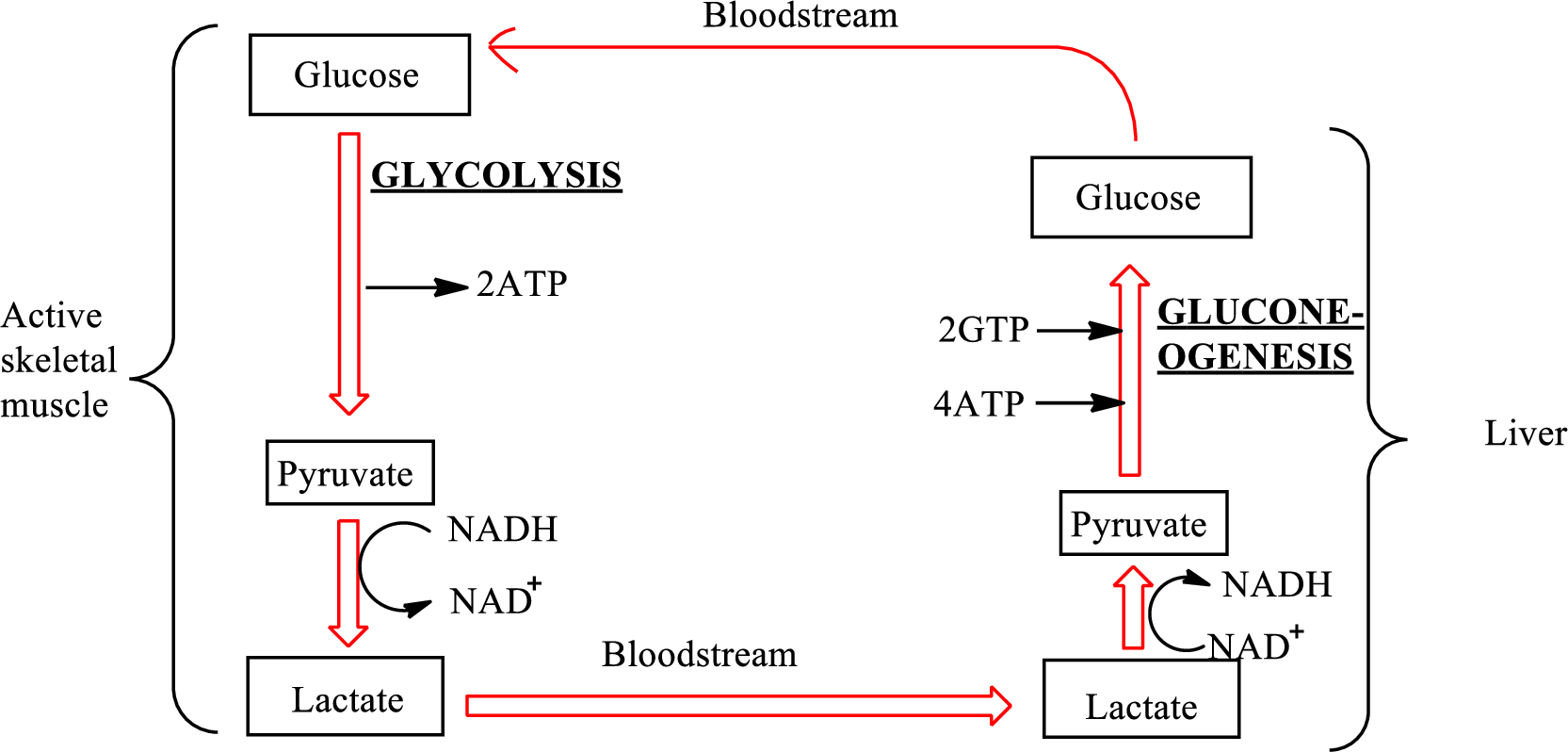
In the Cori cycle,
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation. The chemical reaction for the formation of lactate is as follows:
In the lactate fermentation, NADH is oxidized to
The net overall equation for the glycolysis process is as follows:
(c)
Interpretation: To indicate whether
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
(c)
Answer to Problem 24.105EP
Explanation of Solution
The first stage of the pentose phosphate pathway is the oxidative stage. In the oxidative stage (involves three steps) of the pentose phosphate pathway,
The general equation for the entire pentose phosphate pathway is as follows:
Therefore,
The net overall equation for the glycolysis process is as follows:
Glucose enters the glycolysis metabolic pathway in the form of
(d)
Interpretation: To indicate whether
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
(d)
Answer to Problem 24.105EP
Explanation of Solution
The first stage of the pentose phosphate pathway is the oxidative stage. In the oxidative stage (involves three steps) of the pentose phosphate pathway,
The first step of the second stage i.e. the non-oxidative stage of the pentose phosphate pathway is the isomerization of
Want to see more full solutions like this?
Chapter 24 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





