
Concept explainers
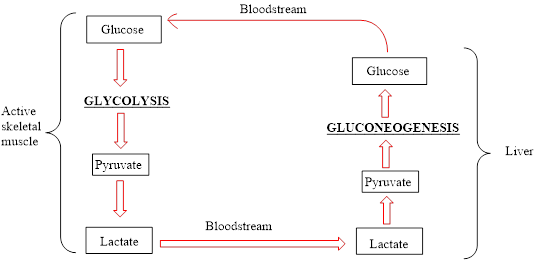
Interpretation: To write an equation for the conversion of lactate to pyruvate in the Cori cycle that includes the structures of lactate and pyruvate.
Concept introduction: Glucose is converted to pyruvate by glycolysis
The Cori cycle is named after its discoverers, Gerty Radnitz Cori, and Carl Cori.
An overview of the Cori cycle is as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- Transcription and Translation 1. What is the main function of transcription and translation? (2 marks) 2. How is transcription different in eukaryotic and prokaryotic cells? (2 marks) 3. Explain the difference between pre-mRNA and post-transcript mRNA. (2 marks) 4. What is the function of the following: (4 marks) i. the cap ii. spliceosome iii. Poly A tail iv. termination sequence 5. What are advantages to the wobble feature of the genetic code? (2 marks) 6. Explain the difference between the: (3 marks) i. A site & P site ii. codon & anticodon iii. gene expression and gene regulation 7. Explain how the stop codon allows for termination. (1 mark) 8. In your own words, summarize the process of translation. (2 marks)arrow_forwardIn this activity you will research performance enhancers that affect the endocrine system or nervous system. You will submit a 1 page paper on one performance enhancer of your choice. Be sure to include: the specific reason for use the alleged results on improving performance how it works how it affect homeostasis and improves performance any side-effects of this substancearrow_forwardNeurons and Reflexes 1. Describe the function of the: a) dendrite b) axon c) cell body d) myelin sheath e) nodes of Ranvier f) Schwann cells g) motor neuron, interneuron and sensory neuron 2. List some simple reflexes. Explain why babies are born with simple reflexes. What are they and why are they necessary. 3. Explain why you only feel pain after a few seconds when you touch something very hot but you have already pulled your hand away. 4. What part of the brain receives sensory information? What part of the brain directs you to move your hand away? 5. In your own words describe how the axon fires.arrow_forward
- Mutations Here is your template DNA strand: CTT TTA TAG TAG ATA CCA CAA AGG 1. Write out the complementary mRNA that matches the DNA above. 2. Write the anticodons and the amino acid sequence. 3. Change the nucleotide in position #15 to C. 4. What type of mutation is this? 5. Repeat steps 1 & 2. 6. How has this change affected the amino acid sequence? 7. Now remove nucleotides 13 through 15. 8. Repeat steps 1 & 2. 9. What type of mutation is this? 0. Do all mutations result in a change in the amino acid sequence? 1. Are all mutations considered bad? 2. The above sequence codes for a genetic disorder called cystic fibrosis (CF). 3. When A is changed to G in position #15, the person does not have CF. When T is changed to C in position #14, the person has the disorder. How could this have originated?arrow_forwardhoose a scientist(s) and research their contribution to our derstanding of DNA structure or replication. Write a one page port and include: their research where they studied and the time period in which they worked their experiments and results the contribution to our understanding of DNA cientists Watson & Crickarrow_forwardhoose a scientist(s) and research their contribution to our derstanding of DNA structure or replication. Write a one page port and include: their research where they studied and the time period in which they worked their experiments and results the contribution to our understanding of DNA cientists Watson & Crickarrow_forward
- 7. Aerobic respiration of a protein that breaks down into 12 molecules of malic acid. Assume there is no other carbon source and no acetyl-CoA. NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here: 3arrow_forwardFor each of the following problems calculate the following: (Week 6-3 Video with 6-1 and 6-2) Consult the total catabolic pathways on the last page as a reference for the following questions. A. How much NADH and FADH2 is produced and fed into the electron transport chain (If any)? B. How much ATP is made from oxidative phosphorylation (OP), if any? Feed the NADH and FADH2 into the electron transport chain: 3ATP/NADH, 2ATP/FADH2 C. How much ATP is made by substrate level phosphorylation (SLP)? D. How much total ATP is made? Add the SLP and OP together. 1. Aerobic respiration using 0.5 mole of glucose? NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here:arrow_forwardAerobic respiration of one lipid molecule. The lipid is composed of one glycerol molecule connected to two fatty acid tails. One fatty acid is 12 carbons long and the other fatty acid is 18 carbons long in the figure below. Use the information below to determine how much ATP will be produced from the glycerol part of the lipid. Then, in part B, determine how much ATP is produced from the 2 fatty acids of the lipid. Finally put the NADH and ATP yields together from the glycerol and fatty acids (part A and B) to determine your total number of ATP produced per lipid. Assume no other carbon source is available. 18 carbons fatty acids 12 carbons glycerol . Glycerol is broken down to glyceraldehyde 3-phosphate, a glycolysis intermediate via the following pathway shown in the figure below. Notice this process costs one ATP but generates one FADH2. Continue generating ATP with glyceraldehyde-3-phosphate using the standard pathway and aerobic respiration. glycerol glycerol-3- phosphate…arrow_forward
- Don't copy the other answerarrow_forward4. Aerobic respiration of 5 mM acetate solution. Assume no other carbon source and that acetate is equivalent to acetyl-CoA. NADH FADH2 OP ATP SLP ATP Total ATP Show your work using dimensional analysis here: 5. Aerobic respiration of 2 mM alpha-ketoglutaric acid solution. Assume no other carbon source. NADH FADH2 OP ATP Show your work using dimensional analysis here: SLP ATP Total ATParrow_forwardBiology You’re going to analyze 5 ul of your PCR product(out of 50 ul) on the gel. How much of 6X DNAloading buffer (dye) are you going to mix with yourPCR product to make final 1X concentration ofloading buffer in the PCR product-loading buffermixture?arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





