
Interpretation:
The structures of fatty acids, triglycerides, phospholipids and steroids should be described.
Concept introduction:
The molecules which contains hydrocarbons and not soluble in water but soluble in nonpolar solvents is known as lipid.
Lipids are classified as:
Triglycerides, Phospholipids and, Steroids and Waxes
Explanation of Solution
The building blocks of fat in our bodies and in food are known as fatty acids. Fatty acid consists of
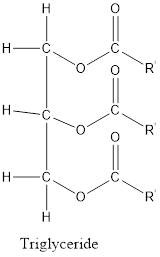
The type of lipid found in our blood is known as triglycerides. Triglyceride is obtained by the three glycerol molecules and three fatty acids. Fats and oils consist of molecules known as triglycerides (contain ester bonds).
In structure of triglyceride, three glycerol molecules and three fatty acids linked with each other by ester bonds.
Structure of triglyceride is:
General structure of phospholipids contains a glycerol molecule(s), phosphate group and two fatty acids. The type of lipid which is the main component of cell membrane is known as phospholipid. Phospholipid consists of two fatty acids and a phosphate group.
In the structure of Phospholipid, two fatty acids and a phosphate group linked with each other by ester bonds.
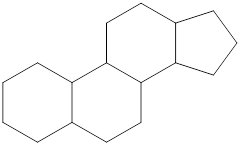
General structure of steroid contains 17 carbon atoms which are linked with each other in four fused rings, three rings contains six carbon atoms that is cyclohexane rings and one is five membered ring that is cyclopentane.
The four ring structure of steroid is:
Chapter 23 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Organic Chemistry (8th Edition)
Applications and Investigations in Earth Science (9th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





