
Interpretation:
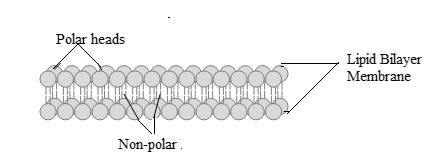
The portion of lipid bilayer membrane should be drawn along with the labeling of polar and nonpolar parts of membrane.
Concept introduction:
The molecules which contains hydrocarbons and not soluble in water but soluble in nonpolar solvents is known as lipid.
Lipids are classified as:
Triglycerides, Phospholipids and, Steroids and Waxes.
The type of lipid found in our blood is known as triglycerides. Triglyceride is obtained by the three glycerol molecules and three fatty acids.
Explanation of Solution
A group of compounds which results in the formation of double-layered surface of all cells is known as membrane lipids. Membrane lipids are classified as: cholesterol, glycolipids and phospholipids. Lipids are amphiphilic in nature which implies that lipids consist of both polar end (soluble in water) and nonpolar end (insoluble in water). The double layer having polar ends are directed outwards and nonpolar ends are directed inwards membrane lipids, and results in the formation of lipid bilayer that separates the interior and exterior of the cell.
Thus, the structure of lipid bilayer membrane with polar heads and non-polar part is:
Chapter 23 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Campbell Biology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Concepts of Genetics (12th Edition)
Introductory Chemistry (6th Edition)
- You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products: xi 1. ☑ 2. H₂O хе i Draw the missing reagent X you think will make this synthesis work in the drawing area below. If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. There is no reagent that will make this synthesis work without complications. : ☐ S ☐arrow_forwardPredict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forward
- For each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forwardPredict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forward
- For each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forward
- give example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





