
Interpretation:
The structures of amino acids, dipeptides, polypeptides and proteins should be compared. The largest molecular mass and smallest molecular mass species should be determined.
Concept introduction:
Proteins are polymeric
They polymerize by peptide linkage to form dipeptide, oligopeptide and polypeptide +
molecules. Each peptide bond formation is a condensation reaction that occurs with the elimination of water molecule.
Explanation of Solution
Amino acids are the organic molecules with
The general structure of amino acid is:
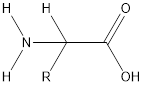
Peptide bonds are identified by the bonding present between carboxyl group of one amino acid and amino group of another amino acid.
Dipeptide is formed when two amino acids reacts with each other and linked by peptide bond.
The general structure of dipeptide is:
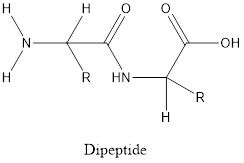
Polypeptides composed of many amino acids linked by peptide bonds arranged in a linear chain.
The polymeric biomolecules which are formed by the polymerization of amino acids are known as proteins. Amino acids are the building block of
Amino acid has smallest molecular mass in comparison to dipeptide, polypeptide and polymers. Dipeptide has largest molecular mass in comparison to an amino acid.
Now, proteins have large molecular mass in comparison to polypeptide as proteins are made up of more amino acids (more than polypeptide) or one or more polypeptides are arranged in a linear chain whereas polypeptides are made up of less than 50 amino acids. Therefore, proteins have large molecular mass in comparison to polypeptide.
The order of molecular mass is:
Chapter 23 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Human Anatomy & Physiology (2nd Edition)
Organic Chemistry (8th Edition)
Concepts of Genetics (12th Edition)
Campbell Biology (11th Edition)
- a. OH H₂N-O -Ph H+ acyclic productarrow_forwardeks.com/aleksogi/x/sl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvTCeeBZbufuBYTI0Hz7m7D3ZS17Hd6m-HIl6n52njJN-TXdQA2X9yID-1SWQJTgnjARg30 111 States of Matter Understanding conceptual components of the enthalpy of solution 0/5 Ge A small amount of acetonitrile (CH, CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketched below. (These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.) CH,CN H₂O B 88 C Use these sketches to answer the questions in the table below. The enthalpy of solution AH is negative soln when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. Would heat be absorbed or released if the system moved from Stage C to D? What force would oppose or favor the system moving from Stage C to D? Check all that apply. 1 absorbed O released neither absorbed nor released. none O ionic bonding force covalent bonding force…arrow_forwardIn a system with an anodic overpotential, the variation of ŋ as a function of the current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Find the range of current densities for which the overpotential has the same value as when calculated for cases 1 and 2 (maximum relative difference of 5% with respect to the behavior for higher fields). To which overpotential range does this correspond? Data: 10 = 1.5 mA cm², T = 300°C, ẞ = 0.64, R = 8.314 J K 1 mol¹ and F = 96485 C mol-1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





