
(a)
Interpretation: For a given set of nitrogen containing compounds, general or IUPAC names have to be assigned.
Concept Introduction: If  ,
, 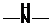 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary
groups are attached to the parent carbon, they are called primary, secondary and tertiary
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc. carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configuration results.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(b)
Interpretation: For a given set of nitrogen containing compounds, general or IUPAC names have to be assigned.
Concept Introduction: If  ,
, 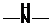 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc. carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc. carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configuration results.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(c)
Interpretation: For a given set of nitrogen containing compounds, general or IUPAC names have to be assigned.
Concept Introduction: If  ,
, 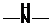 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc. carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc. carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configuration results.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(d)
Interpretation: For a given set of nitrogen containing compounds, general or IUPAC names have to be assigned.
Concept Introduction: If  ,
, 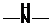 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc. carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc. carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configuration results.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(e)
Interpretation: For a given set of nitrogen containing compounds, general or IUPAC names have to be assigned.
Concept Introduction: If  ,
, 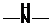 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc. carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc. carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configuration results.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(f)
Interpretation: For a given set of nitrogen containing compounds, general or IUPAC names have to be assigned.
Concept Introduction: If  ,
, 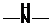 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc. carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc. carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configuration results.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry, Binder Ready Version
- Draw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardIdentify each chiral carbon as either R or S. Identify the overall carbohydrates as L or Darrow_forward
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





