
(a)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 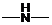 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary
groups are attached to the parent carbon, they are called primary, secondary and tertiary
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
(b)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 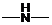 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
In the given compound (b), three alkyl groups are attached to nitrogen atom. Therefore, it belongs to tertiary amine.
Decide the given name which is in general name method or IUPAC name method
There are three alkyl groups present in the given name. Here, the main chain is cyclopropylamine. Three-membered carbon ring structure is called cyclopropyl group. So, general name system is followed.
Locate the substituents and draw the corresponding structure for the given name
(c)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 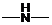 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
In the given compound (c), only one alkyl group, namely pentane is attached to nitrogen atom. Therefore, it belongs to primary amine.
Decide the given name which is in general name method or IUPAC name method
There is only one alkane group present in the given name. It is pentane attached to primary amine named as pentanamine which is the main chain of the given name. So, IUPAC name system is followed.
Locate the substituents and draw the corresponding structure for the given name
(d)
Interpretation: For a given set of nitrogen containing compounds, the structures have to be drawn using their general or IUPAC names.
Concept Introduction: If  ,
, 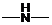 and
and  groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
groups are attached to the parent carbon, they are called primary, secondary and tertiary amines respectively.
There are two ways followed to name the compound. First one is the method of giving general name in which name of the alkyl group followed by amine name is given. Second one is the method giving IUPAC name in which name of the alkane group followed by amine name is given.
The length of the chain which is having more number of carbon atoms is selected as the parent or main chain. Other chains are considered as substituents to the main chain. Position of the substituents should be included in the name. If one, two, three, four, five, six, etc carbons are activating as the main chain in IUPAC system, then the name of the compound comes as methane, ethane, propane, butane, pentane, hexane, etc. which are the name of alkanes. If one, two, three, four, five, six, etc carbons are activating as the main chain in general name method, then the name of the compound comes as methyl, ethyl, propyl, butyl, pentyl, hexyl, etc. which are the name of alkyl groups. If substituent groups are attached to nitrogen atom as in the case of tertiary or secondary amines, the name is given as N-alkyl name of the substituent.
If any configuration is present in the compound, that should be assigned to it while writing the name. If the compound contains heavier groups on the same side, it gets (Z)-configuration. If they are on the opposite directions, (E)-configurations result.
If a carbon has four different groups attached to it, that carbon shows a chirality nature. If that chiral carbon rotates the plane polarized light into a clockwise direction, it gets (R)-isomer. If that carbon rotates the plane polarized light into a counter-clockwise direction, it gets (S)-isomer.
To find: Categorize the number of alkyl groups attached to nitrogen atom
In the given compound (d), only one alkyl group is attached to nitrogen atom. Therefore, it belongs to primary amine.
Decide the given name which is in general name method or IUPAC name method
There is only one alkyl group present in the given name. It is benzyl group. So, simple general name system is followed. If phenyl group is attached to methylene group, it is called benzyl group.
Locate the substituents and draw the corresponding structure for the given name
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry, Binder Ready Version
- Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forward
- Predict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forward
- Draw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forward
- What is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





