
(a)
Interpretation: The products of the reactions in which diazonium salt is treated with the given reagents have to be drawn.
Concept Introduction:
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
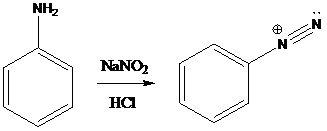
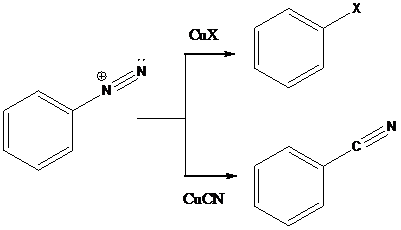
Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.
Schiemann reaction is the reaction in which diazonium salt is converted into fluoro group using fluoroboric acid (HBF4).
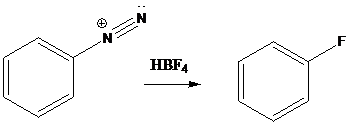
To find: Get the product of the reaction in which diazonium salt is treated with the given reagent (a)
Put the reaction of m-bromoaniline with NaNO2 and HCl
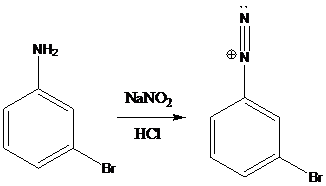
(b)
Interpretation: The products of the reactions in which diazonium salt is treated with the given reagents have to be drawn.
Concept Introduction:
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
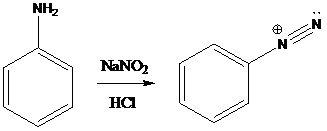
Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

Schiemann reaction is the reaction in which diazonium salt is converted into fluoro group using fluoroboric acid (HBF4).
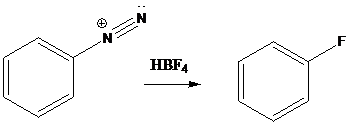
To find: Get the product of the reaction in which diazonium salt is treated with the given reagent (b)
Put the reaction of m-bromoaniline with NaNO2 and HCl
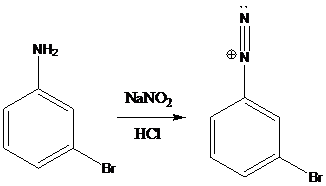
(c)
Interpretation: The products of the reactions in which diazonium salt is treated with the given reagents have to be drawn.
Concept Introduction:
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
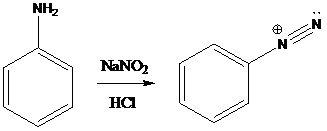
Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

Schiemann reaction is the reaction in which diazonium salt is converted into fluoro group using fluoroboric acid (HBF4).
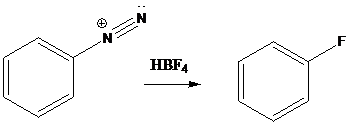
To find: Get the product of the reaction in which diazonium salt is treated with the given reagent
Put the reaction of m-bromoaniline with NaNO2 and HCl
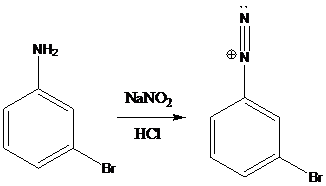
(d)
Interpretation: The products of the reactions in which diazonium salt is treated with the given reagents have to be drawn.
Concept Introduction:
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
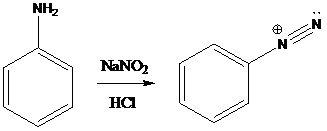
Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

Schiemann reaction is the reaction in which diazonium salt is converted into fluoro group using fluoroboric acid (HBF4).
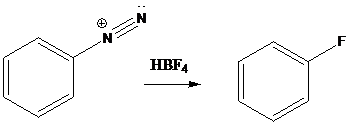
To find: Get the product of the reaction in which diazonium salt is treated with the given reagent
Put the reaction of m-bromoaniline with NaNO2 and HCl
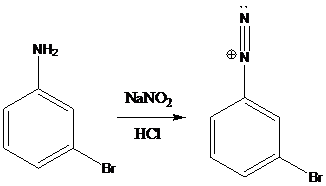
(e)
Interpretation: The products of the reactions in which diazonium salt is treated with the given reagents have to be drawn.
Concept Introduction:
A compound containing an amino group is treated with sodium nitrite and HCl leading to the formation of diazonium salt.
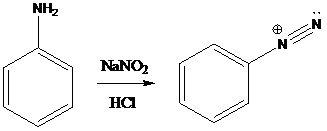
Sandmeyer reactions use copper salts as the reagents. Here, aryldiazonium salt is converted into aryl halides or aryl cyanides by using copper halides or copper cyanides.

Schiemann reaction is the reaction in which diazonium salt is converted into fluoro group using fluoroboric acid (HBF4).
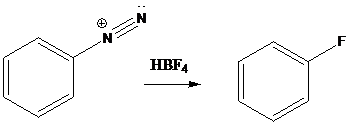
To find: Get the product of the reaction in which diazonium salt is treated with the given reagent (e)
Put the reaction of m-bromoaniline with NaNO2 and HCl
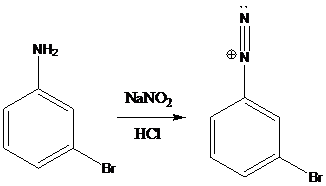
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry, Binder Ready Version
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





