
(a)
Interpretation: The structures of 2 and 3, an acid-catalyzed mechanism for the conversion of 3 to 4 and the reasons for the equilibrium favor enamine 4 over imine 3 have to be found for the given reactions.
Concept Introduction:
An azide is converted into an
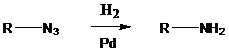
To find: Draw the structures of 2 and 3
Do hydrogenation on an azide
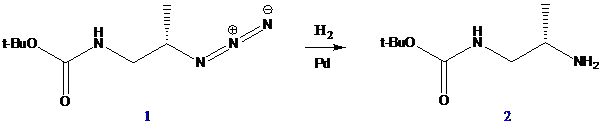
(b)
Interpretation: The structures of 2 and 3, an acid-catalyzed mechanism for the conversion of 3 to 4 and the reasons for the equilibrium favor enamine 4 over imine 3 have to be found for the given reactions.
Concept Introduction:
An enamine is an unsaturated compound derived by the reaction of an
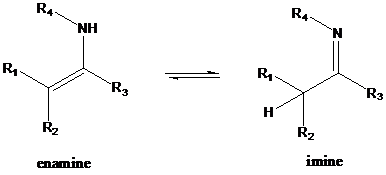
To find: Provide an acid-catalyzed mechanism for the conversion of 3 to 4
Do protonation for imine

(c)
Interpretation: The structures of 2 and 3, an acid-catalyzed mechanism for the conversion of 3 to 4 and the reasons for the equilibrium favor enamine 4 over imine 3 have to be found for the given reactions.
Concept Introduction:
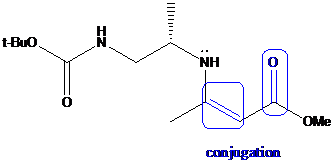
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O. The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group containing both alkene (en-) and alcohol (-ol). Enamines are considered to be nitrogen analogs of enols. If one of the nitrogen substituents is a hydrogen atom, H, it is the tautomeric form of an imine. This usually will rearrange to the imine; however there are several exceptions (such as aniline). The enamine-imine tautomerism may be considered analogous to the keto-enol tautomerism. In both cases, a hydrogen atom switches its location between the heteroatom (oxygen or nitrogen) and the second carbon atom. Enamines are both good nucleophiles and good bases.

To find: Get the reasons for the equilibrium favor enamine 4 over imine 3
Explain the conjugation
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry, Binder Ready Version
- Add substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. + I I H CH3 Ph Досн Br OCH 3 Drawing Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo Reset Remove Done Rotatearrow_forward20.17 Predict the structure of the major product formed by 1,2-addition of HBr to 3-methylenecyclohexene. 3-Methylenecyclohexene 20.18 Predict the major product formed by 1,4-addition of HBr to 3-methylenecyclohexene.arrow_forward+ Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. Br Drawing Strong Base H Q Atoms, Bonds Charges and Rings Draw or tap a new bond to see suggestions. Remove Done 語 Reset Undo + Drag To Panarrow_forward
- Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. + Drawing Į Strong Base H Br Q Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Reset 謂 Remove Done Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. + Br CH3 Q Strong Base Drawing Atoms, Bonds and Rings Charges Undo Reset H "Br H N Br. Remove Done .N. Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this elementary step in an elimination mechanism. Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore byproducts. + Br: .. 8 0.01 M NaOH heat Drawing Q Atoms, Bonds and Rings Charges and Lone Pairs Draw or tap a new bond to see suggestions. Undo Reset Remove Done + Drag To Panarrow_forward
- + Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. Ph CH2CH3 H H3C H Br DBN [૪] Drawing Atoms, Bonds and Rings H | OH Charges ―00 H. C | Undo Reset Br I Remove Done Drag To Pan +arrow_forwardReaction A Now the production A Œ In the product of reaction i 12 Dear the product of actionarrow_forwardMacmillan Learnin When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm-1. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 DEPT 135 200 160 120 80 40 0 Draw the unknown amide. 40 40 0arrow_forward
- Draw the major product karmed when I reach with the epoxide. Use walge dah bonds, including hydrogen al alcach genic center, to show the chemistry of the product Beeldraw any hydrogen akams on coxygen where applicablearrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H I Select to Add Arrows + H H 'H Q H2O H2O CI:O .H H H H I Select to Add Arrows I : C H2O H H H Select to Add Arrows 'Harrow_forward+ Draw an alkyl halide that produces ONLY the following alkene in an E2 elimination. Ignore any inorganic byproducts. Drawing Strong Base Q Atoms, Bonds and Rings Charges HO Br H2N Undo Reset Remove Done Drag To Panarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





