
(a)
Interpretation:
Classification of the reaction should be identified for the given transformation.
Concept introduction:
Substitution reaction: During a
Nucleophilic Substitution reaction: electron rich nucleophile attacks the positive or partially positive charge of an atom and replace a leaving group is called Nucleophilic Substitution reaction.
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms
Addition reaction: addition reaction is two or more molecules combine to form a larger one.
Isomerization: one molecule is transformed into another molecule with same molecular formula but different arrangement.
(a)
Answer to Problem 23.52QP
The given reaction is isomerization reaction, the reaction is shown below (a)
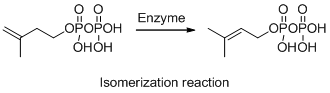
Explanation of Solution
To find:type of the reaction.
The given reaction is isomerization reaction, the reaction is shown below
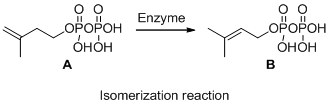
Compound (A) is transformed into compound (B) with same molecular formula but different arrangement so it is called isomerization. The reaction is called isomerization.
(b)
Interpretation:
Classification of the reaction should be identified for the given transformation.
Concept introduction:
Substitution reaction: During a chemical reaction when one functional group is transformed as another functional group in a chemical compound is called substitution reaction.
Nucleophilic Substitution reaction: electron rich nucleophile attacks the positive or partially positive charge of an atom and replace a leaving group is called Nucleophilic Substitution reaction.
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E1 reaction. When two substituents are removed from the molecule in two steps is called E2 reaction.
Addition reaction: addition reaction is two or more molecules combine to form a larger one.
Isomerization: one molecule is transformed into another molecule with same molecular formula but different arrangement.
(b)
Answer to Problem 23.52QP
The given reaction is substitution reaction, the reaction is shown below (b)
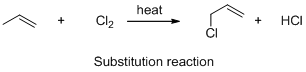
Explanation of Solution
To find: type of the reaction.
The given reaction is substitution reaction, the reaction is shown below
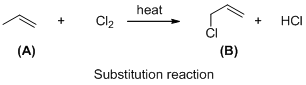
Compound (A) is undergoes reaction with chlorine which forms chlorinatedcompound (B) with elimination of hydrochloric acid in the presence of heat and the hydrogen atom is replaced by the chlorine atom. The reaction is called substitution reaction.
(c)
Interpretation:
Classification of the reaction should be identified for the given transformation.
Concept introduction:
Substitution reaction: During a chemical reaction when one functional group is transformed as another functional group in a chemical compound is called substitution reaction.
Nucleophilic Substitution reaction: electron rich nucleophile attacks the positive or partially positive charge of an atom and replace a leaving group is called Nucleophilic Substitution reaction.
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E1 reaction. When two substituents are removed from the molecule in two steps is called E2 reaction.
Addition reaction: addition reaction is two or more molecules combine to form a larger one.
Isomerization: one molecule is transformed into another molecule with same molecular formula but different arrangement.
(c)
Answer to Problem 23.52QP
The given reaction is addition reaction, the reaction is shown below (c)
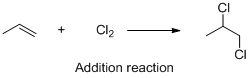
Explanation of Solution
To find: type of the reaction.
The given reaction is addition reaction, the reaction is shown below
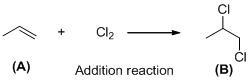
Compound (A) is undergoes addition reaction with chlorine which forms chlorinated compound (B). The reaction is called addition reaction.
(d)
Interpretation:
Classification of the reaction should be identified for the given transformation.
Concept introduction:
Substitution reaction: During a chemical reaction when one functional group is transformed as another functional group in a chemical compound is called substitution reaction.
Nucleophilic Substitution reaction: electron rich nucleophile attacks the positive or partially positive charge of an atom and replace a leaving group is called Nucleophilic Substitution reaction.
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E1 reaction. When two substituents are removed from the molecule in two steps is called E2 reaction.
Addition reaction: addition reaction is two or more molecules combine to form a larger one.
Isomerization: one molecule is transformed into another molecule with same molecular formula but different arrangement.
(d)
Answer to Problem 23.52QP
The given reaction is substitution reaction, the reaction is shown below (d)
Explanation of Solution
To find: type of the reaction.
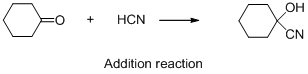
The given reaction is addition reaction, the reaction is shown below (e)
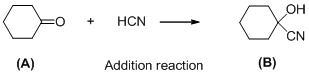
Compound (A) is undergoes reaction with hydrogen cyanide which forms cyanohydrin (B). The reaction is called addition reaction.
(e)
Interpretation:
Classification of the reaction should be identified for the given transformation.
Concept introduction:
Substitution reaction: During a chemical reaction when one functional group is transformed as another functional group in a chemical compound is called substitution reaction.
Nucleophilic Substitution reaction: electron rich nucleophile attacks the positive or partially positive charge of an atom and replace a leaving group is called Nucleophilic Substitution reaction.
Elimination reaction: An elimination reaction is removal of two substituents in a molecule and forms alkene. An elimination reaction is one or two-step process which based on the mechanism when two substituents removed from the molecule in single step is called E1 reaction. When two substituents are removed from the molecule in two steps is called E2 reaction.
Addition reaction: addition reaction is two or more molecules combine to form a larger one.
Isomerization: one molecule is transformed into another molecule with same molecular formula but different arrangement.
(e)
Answer to Problem 23.52QP
The given reaction is addition reaction, the reaction is shown below (e)

The given reaction is elimination reaction, the reaction is shown below (e)
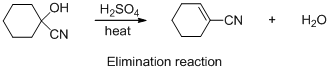
Explanation of Solution
To find: type of the reaction.
The given reaction is elimination reaction, the reaction is shown below
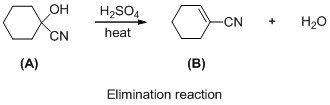
Compound (A) is undergoes elimination reaction with sulfuric acid which forms corresponding elimination compound (B). The reaction is called elimination reaction.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Atoms First
- + C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward→ Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forward
- For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardIdentifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Assign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forwarddescrive the energy levels of an atom and howan electron moces between themarrow_forwardRank each set of substituents using the Cahn-Ingold-Perlog sequence rules (priority) by numbering the highest priority substituent 1.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





