
Concept explainers
(a)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
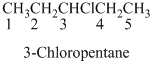
If the molecules having
The given compound is an alcohol
Example is given below
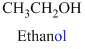
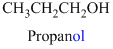
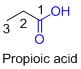
The given compound is an acid (
The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
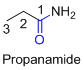
If the molecule is ester,
Esters end with “ate”
Example
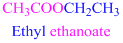
The given compound is an
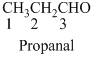
The given compound is an aldehyde (
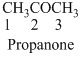
The given compound is an aldehyde (
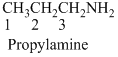
(a)
Answer to Problem 23.19QP
Answer
- (1) Structure of the given organic compounds is shown below (a).
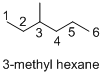
Explanation of Solution
To find: The structure of the given organic molecule.
Name of the given organic compounds is 3-methyl hexane.

Parent chain is identified and numbering is given for the compound. According to the name, third carbon is bearing one methyl group and the given name is hexane which clearly indicates that the compound having six carbon atoms in the molecule. According to the name, the structure of the molecule is given above.
(b)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (4) Name the parent alkane (long alkyl chain)
- (5) Number the carbon
- (6) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
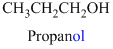
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
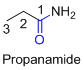
If the molecule is ester,
Esters end with “ate”
Example
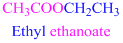
The given compound is an aldehyde (
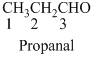
The given compound is an aldehyde (
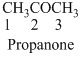
The given compound is an aldehyde (
(b)
Answer to Problem 23.19QP
Answer
Structure of the given organic compounds is shown below (b).
Explanation of Solution
To find: The structure of the given organic molecule.
Name of the given organic compounds is 2,3-dimethylpentane
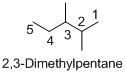
Parent chain is identified and numbering is given for the compound. According to the name, second and third carbon is bearing methyl group and the parent name of the molecule is pentane which clearly indicates that the compound having five carbon atoms in the parent. According to the name, the structure of the molecule is given above.
(c)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (7) Name the parent alkane (long alkyl chain)
- (8) Number the carbon
- (9) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
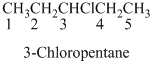
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
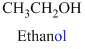
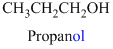
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
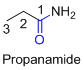
If the molecule is ester,
Esters end with “ate”
Example
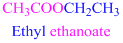
The given compound is an aldehyde (
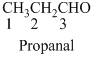
The given compound is an aldehyde (
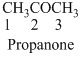
The given compound is an aldehyde (
(c)
Answer to Problem 23.19QP
Structure of the given organic compounds is shown below (c).
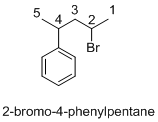
Explanation of Solution
To find: The structure of the given organic molecule.
Name of the given organic compounds is 2-bromo-4-phenylpentane.

Parent chain is identified and numbering is given for the compound. According to the name, second carbon is bearing bromine and fourth carbon is bearing phenyl ring and the parent name of the molecule is pentane which clearly indicates that the compound having five carbon atoms in the parent. According to the name, the structure of the molecule is given above.
(d)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (10) Name the parent alkane (long alkyl chain)
- (11) Number the carbon
- (12) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
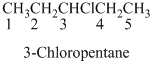
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
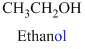
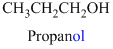
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
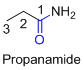
If the molecule is ester,
Esters end with “ate”
Example
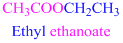
The given compound is an aldehyde (
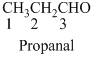
The given compound is an aldehyde (
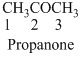
The given compound is an aldehyde (
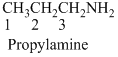
(d)
Answer to Problem 23.19QP
Answer
Structure of the given organic compounds is shown below (d).

Explanation of Solution
To find: The structure of the given organic molecule.
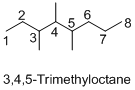
Name of the given organic compounds is 3,4,5-trimethyloctane.

Parent chain is identified and numbering is given for the compound. According to the name, third, fourth and fifth carbon is bearing methyl group and the parent name of the molecule is octane which clearly indicates that the compound having eight carbon atoms in the parent. According to the name, the structure of the molecule is given above.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Atoms First
- Fill-in-the molecules for the oxidation or reduction of the starting alcohol.arrow_forwardName the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


