
Concept explainers
(a)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
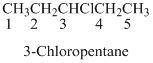
If the molecules having
The given compound is an alcohol
Example is given below
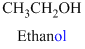
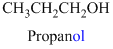
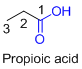
The given compound is an acid (
The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
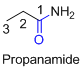
If the molecule is ester,
Esters end with “ate”
Example
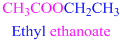
The given compound is an
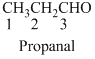
The given compound is a
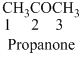
The given compound is an
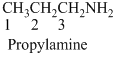
(a)
Answer to Problem 23.9QP
Answer
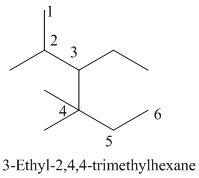
- (1) Name of the given organic compounds is shown below (a).
Explanation of Solution
To find: The name of the given organic compounds
Name of the given organic compounds is shown below.

Parent chain is identified and numbering is given for the compound.
Second, fourth carbon is bearing one and two methyl group and it has six carbon atom in the molecule. The name of the given organic compound is 3-Ethyl-2,4,4-trimethylhexane.
(b)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.

If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
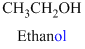
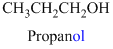
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
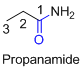
If the molecule is ester,
Esters end with “ate”
Example
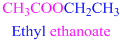
The given compound is an aldehyde (
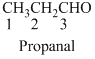
The given compound is a ketone (
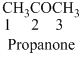
The given compound is an amine (
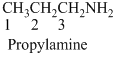
(b)
Answer to Problem 23.9QP
Answer
Name of the given organic compounds is shown below (b)
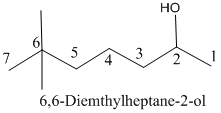
Explanation of Solution
To find:The name of the given organic compounds
Name of the given organic compounds is shown below.

Parent chain is identified and numbering is given for the compound.
sixth carbon is bearing two methyl group and it has seven carbon atom in the molecule. The name of the given organic compound is 6,6-dimethylheptane-2-ol.
(c)
Interpretation:
The structure of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.

If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
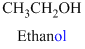
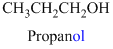
The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
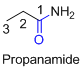
If the molecule is ester,
Esters end with “ate”
Example
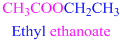
The given compound is an aldehyde (
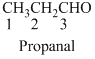
The given compound is a ketone (
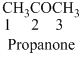
The given compound is an amine (
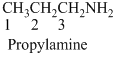
(c)
Answer to Problem 23.9QP
Answer
Name of the given organic compounds is shown below (c)
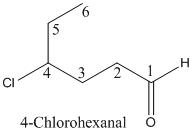
Explanation of Solution
To find:The name of the given organic compounds
Name of the given organic compounds is shown below.

Parent chain is identified and numbering is given for the compound.
First carbon is bearing aldehyde and fourth carbon bearing chlorine (halogen) group and it has six carbon atoms in the molecule. The name of the given organic compound is 4-Chlorohexanal.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: Atoms First
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forward
- Draw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward
- 3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forwardWrite the aldol condensation mechanism and product for benzaldehyde + cyclohexanone in a base. Then trans-cinnamaldehyde + acetone in base. Then, trans-cinnamaldehyde + cyclohexanone in a base.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





