
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22.SE, Problem 37AP
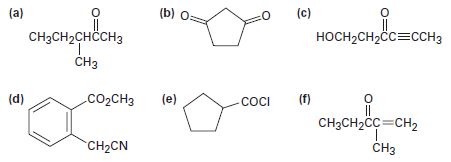
Identify all the acidic hydrogens (pKa < 25) in the following molecules:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
22
PLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS FOR THE MECHANISM!!! THANKS
First image: QUESTION 6. I have to show, with ARROWS and STRUCTURES, the mechanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary.
I also tried to draw the mechanism, tell me what to change. Please note that its an AMIDE thats formed not an AMINE the nitrogen has ONE hydrogen and one Phenyl-C-Phenyl. I already asked for this mechanism and got as a final product ...-NH2 not whats shown on the picture, thank you
Ths second part. QUESTION 3. I just need a way to synthesize the lactone A, I already started please continue from where I left it
Second image: I simply need the products, substrates or reagents, thank you
Indicate how to prepare a 10% sodium hydroxide (NaOH) solution to a slightly alkaline pH.
Chapter 22 Solutions
Organic Chemistry
Ch. 22.1 - Prob. 1PCh. 22.1 - How many acidic hydrogens does each of the...Ch. 22.1 - Prob. 3PCh. 22.3 - Write the complete mechanism for the deuteration...Ch. 22.3 - Prob. 5PCh. 22.4 - If methanol rather than water is added at the end...Ch. 22.5 - Prob. 7PCh. 22.5 - Draw a resonance structure of the acetonitrile...Ch. 22.6 - If methanol rather than water is added at the end...Ch. 22.7 - Prob. 10P
Ch. 22.7 - Draw a resonance structure of the acetonitrile...Ch. 22.7 - Why do you suppose ketone halogenations in acidic...Ch. 22.7 - Prob. 13PCh. 22.7 - Prob. 14PCh. 22.7 - Prob. 15PCh. 22.7 - Prob. 16PCh. 22.SE - Prob. 17VCCh. 22.SE - Prob. 18VCCh. 22.SE - Prob. 19VCCh. 22.SE - Prob. 20MPCh. 22.SE - Predict the product(s) and provide the mechanism...Ch. 22.SE - Predict the product(s) and provide the mechanism...Ch. 22.SE - Prob. 23MPCh. 22.SE - In the Hell–Volhard–Zelinskii reaction, only a...Ch. 22.SE - Prob. 25MPCh. 22.SE - Nonconjugated , -unsaturated ketones, such as...Ch. 22.SE - Prob. 27MPCh. 22.SE - Using curved arrows, propose a mechanism for the...Ch. 22.SE - Prob. 29MPCh. 22.SE - One of the later steps in glucose biosynthesis is...Ch. 22.SE - The Favorskii reaction involves treatment of an...Ch. 22.SE - Treatment of a cyclic ketone with diazomethane is...Ch. 22.SE - Prob. 33MPCh. 22.SE - Amino acids can be prepared by reaction of alkyl...Ch. 22.SE - Amino acids can also be prepared by a two-step...Ch. 22.SE - Heating carvone with aqueous sulfuric acid...Ch. 22.SE - Identify all the acidic hydrogens (pKa 25) in the...Ch. 22.SE - Rank the following compounds in order of...Ch. 22.SE - Prob. 39APCh. 22.SE - Base treatment of the following , -unsaturated...Ch. 22.SE - Prob. 41APCh. 22.SE - Prob. 42APCh. 22.SE - Prob. 43APCh. 22.SE - Which, if any, of the following compounds can be...Ch. 22.SE - Prob. 45APCh. 22.SE - Prob. 46APCh. 22.SE - Prob. 47APCh. 22.SE - How might you convert geraniol into either ethyl...Ch. 22.SE - Prob. 49APCh. 22.SE - One way to determine the number of acidic...Ch. 22.SE - Prob. 51APCh. 22.SE - Prob. 52APCh. 22.SE - Prob. 53APCh. 22.SE - Prob. 54APCh. 22.SE - Prob. 55APCh. 22.SE - Prob. 56APCh. 22.SE - All attempts to isolate primary and secondary...Ch. 22.SE - How would you synthesize the following compounds...Ch. 22.SE - Prob. 59APCh. 22.SE - Prob. 60APCh. 22.SE - Prob. 61APCh. 22.SE - Prob. 62APCh. 22.SE - As far back as the 16th century, South American...Ch. 22.SE - The key step in a reported laboratory synthesis of...Ch. 22.SE - Prob. 65AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH, CH CH₂ CH₂ Phytyl side chain 5. What is the expected order of elution of compounds A-D below from a chromatography column packed with silica gel, eluting with hexane/ethyl acetate? C D OHarrow_forwardPlease analze my gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Attached is the following image for the order of column wells and my gel.arrow_forward2.0arrow_forward
- Write the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 5 6 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! ☐arrow_forwardCompare these chromatograms of three anti-psychotic drugs done by HPLC and SFC. Why is there the difference in separation time for SFC versus HPLC? Hint, use the Van Deemter plot as a guide in answering this question. Why, fundamentally, would you expect a faster separation for SFC than HPLC, in general?arrow_forwardA certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward
- 2.arrow_forwardPlease solve for the following Electrochemistry that occursarrow_forwardCommercial bleach contains either chlorine or oxygen as an active ingredient. A commercial oxygenated bleach is much safer to handle and less likely to ruin your clothes. It is possible to determine the amount of active ingredient in an oxygenated bleach product by performing a redox titration. The balance reaction for such a titration is: 6H+ +5H2O2 +2MnO4- à 5O2 + 2Mn2+ + 8H2O If you performed the following procedure: “First, dilute the Seventh Generation Non-Chlorine Bleach by pipetting 10 mL of bleach in a 100 mL volumetric flask and filling the flask to the mark with distilled water. Next, pipet 10 mL of the diluted bleach solution into a 250 mL Erlenmeyer flask and add 20 mL of 1.0 M H2SO4 to the flask. This solution should be titrated with 0.0100 M KMnO4 solution.” It took 18.47mL of the KMnO4 to reach the endpoint on average. What was the concentration of H2O2 in the original bleach solution in weight % assuming the density of bleach is 1g/mL?arrow_forward
- 10.arrow_forwardProper care of pH electrodes: Why can you not store a pH electrode in distilled water? What must you instead store it in? Why?arrow_forwardWrite the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 569 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! §arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY