
a)
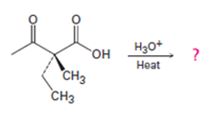
Interpretation:
Whether the
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
b)
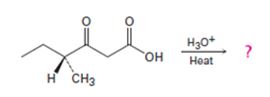
Interpretation:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not is to be stated. A mechanism to explain the formation of the ketone is to be proposed.
Concept introduction:
The decarboxylation of a β- keto acid takes place through the formation of a planar enol intermediate which then tautomerizes to the keto form. If the β- keto acid is optically active and if the chiral centre is involved in the reaction, the ketone product will be racemic and optically inactive. If the chiral centre is not involved in the reaction then the optical activity is retained in the ketone product.
To state:
Whether the ketone obtained by the decarboxylation of the optically active β- keto acid will be optically active or not.
To propose:
A mechanism to explain the formation of the ketone.
Trending nowThis is a popular solution!

Chapter 22 Solutions
Organic Chemistry
- Heating an alcohol in the presence of sulfuric or phosphoric acid will cause a dehydration to occur: the removal of the elements of water from a molecule, forming an alkene. The reaction usually follows an E1 mechanism. The SN1 pathway is suppressed by using a strong acid whose conjugate base is a poor nucleophile. Further, heating the reaction mixture causes a greater increase in the rate of E1 compared to the rate of S№1. 2nd attempt 0 See Periodic Table See Hint Draw the organic intermediate from the first step (no byproducts) and draw curved arrow(s) to show how it reacts. TH +11: 1st attempt Feedback H H H C F F See Periodic Table See Hintarrow_forwardThis molecule undergoes an E1 mechanism when stirred in methanol. 3rd attempt CH₂OH CH₂OH 6148 O See Periodic Table. See Hint Draw 3 chemical species including formal charges and lone pairs of electrons. Add the missing curved arrow notation. H N O O SA 3 Br Iarrow_forwardComplete the mechanism for the E1 reaction below by following the directions written above each of the five boxes. Be sure to include lone pair electrons and nonzero formal charges. 1st attempt Y 0 + Provide the missing curved arrow notation. 01: See Periodic Table See Hint H C Br Iarrow_forward
- Please help answer number 2. Thanks in advance.arrow_forwardHow do I explain this? Thank you!arrow_forwardWhen an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 40 0 DEPT 135 T 200 160 120 80 40 0 Draw the unknown amide. Select Dow Templates More Fragearrow_forward
- Identify the unknown compound from its IR and proton NMR spectra. C4H6O: 'H NMR: 82.43 (1H, t, J = 2 Hz); 8 3.41 (3H, s); 8 4.10 (2H, d, J = 2 Hz) IR: 2125, 3300 cm¹ The C4H6O compound liberates a gas when treated with C2H5 MgBr. Draw the unknown compound. Select Draw с H Templates Morearrow_forwardPlease help with number 6 I got a negative number could that be right?arrow_forward1,4-Dimethyl-1,3-cyclohexadiene can undergo 1,2- or 1,4-addition with hydrogen halides. (a) 1,2-Addition i. Draw the carbocation intermediate(s) formed during the 1,2-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,2-addition product formed during the reaction in (i)? (b) 1,4-Addition i. Draw the carbocation intermediate(s) formed during the 1,4-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,4-addition product formed from the reaction in (i)? (c) What is the kinetic product from the reaction of one mole of hydrobromic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (d) What is the thermodynamic product from the reaction of one mole of hydrobro-mic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (e) What major product will result when 1,4-dimethyl-1,3-cyclohexadiene is treated with one mole of hydrobromic acid at - 78 deg * C ? Explain your reasoning.arrow_forward
- Give the product of the bimolecular elimination from each of the isomeric halogenated compounds. Reaction A Reaction B. КОВ CH₂ HotBu +B+ ко HOIBU +Br+ Templates More QQQ Select Cv Templates More Cras QQQ One of these compounds undergoes elimination 50x faster than the other. Which one and why? Reaction A because the conformation needed for elimination places the phenyl groups and to each other Reaction A because the conformation needed for elimination places the phenyl groups gauche to each other. ◇ Reaction B because the conformation needed for elimination places the phenyl groups gach to each other. Reaction B because the conformation needed for elimination places the phenyl groups anti to each other.arrow_forwardFive isomeric alkenes. A through each undergo catalytic hydrogenation to give 2-methylpentane The IR spectra of these five alkenes have the key absorptions (in cm Compound Compound A –912. (§), 994 (5), 1643 (%), 3077 (1) Compound B 833 (3), 1667 (W), 3050 (weak shoulder on C-Habsorption) Compound C Compound D) –714 (5), 1665 (w), 3010 (m) 885 (3), 1650 (m), 3086 (m) 967 (5), no aharption 1600 to 1700, 3040 (m) Compound K Match each compound to the data presented. Compound A Compound B Compound C Compound D Compoundarrow_forward7. The three sets of replicate results below were accumulated for the analysis of the same sample. Pool these data to obtain the most efficient estimate of the mean analyte content and the standard deviation. Lead content/ppm: Set 1 Set 2 Set 3 1. 9.76 9.87 9.85 2. 9.42 9.64 9.91 3. 9.53 9.71 9.42 9.81 9.49arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


