
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22.3, Problem 5P
Interpretation Introduction
Interpretation:
The method for preparation of 1-penten-3-one from 3-pentanone is given.
Concept introduction:
When the α-Bromo
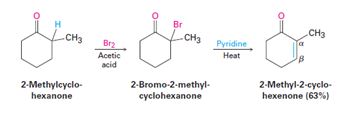
To give:
The preparation method of1-penten-3-one from 3-pentanone is given.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
pls help
Indicate the compound resulting from the bromination of 3,5-dimethylpyrazole
31) The reaction profile for a given chemical reaction is shown below
A
Energy
Reactants
Products
B
Progress of reaction
a. Which arrow represents the activation energy for the forward reaction?
b. Which arrow represents the activation energy for the backward reaction?
c. Is the forward reaction exothermic or endothermic?
d. Is the reverse reaction exothermic or endothermic?
Chapter 22 Solutions
Organic Chemistry
Ch. 22.1 - Prob. 1PCh. 22.1 - How many acidic hydrogens does each of the...Ch. 22.1 - Prob. 3PCh. 22.3 - Write the complete mechanism for the deuteration...Ch. 22.3 - Prob. 5PCh. 22.4 - If methanol rather than water is added at the end...Ch. 22.5 - Prob. 7PCh. 22.5 - Draw a resonance structure of the acetonitrile...Ch. 22.6 - If methanol rather than water is added at the end...Ch. 22.7 - Prob. 10P
Ch. 22.7 - Draw a resonance structure of the acetonitrile...Ch. 22.7 - Why do you suppose ketone halogenations in acidic...Ch. 22.7 - Prob. 13PCh. 22.7 - Prob. 14PCh. 22.7 - Prob. 15PCh. 22.7 - Prob. 16PCh. 22.SE - Prob. 17VCCh. 22.SE - Prob. 18VCCh. 22.SE - Prob. 19VCCh. 22.SE - Prob. 20MPCh. 22.SE - Predict the product(s) and provide the mechanism...Ch. 22.SE - Predict the product(s) and provide the mechanism...Ch. 22.SE - Prob. 23MPCh. 22.SE - In the Hell–Volhard–Zelinskii reaction, only a...Ch. 22.SE - Prob. 25MPCh. 22.SE - Nonconjugated , -unsaturated ketones, such as...Ch. 22.SE - Prob. 27MPCh. 22.SE - Using curved arrows, propose a mechanism for the...Ch. 22.SE - Prob. 29MPCh. 22.SE - One of the later steps in glucose biosynthesis is...Ch. 22.SE - The Favorskii reaction involves treatment of an...Ch. 22.SE - Treatment of a cyclic ketone with diazomethane is...Ch. 22.SE - Prob. 33MPCh. 22.SE - Amino acids can be prepared by reaction of alkyl...Ch. 22.SE - Amino acids can also be prepared by a two-step...Ch. 22.SE - Heating carvone with aqueous sulfuric acid...Ch. 22.SE - Identify all the acidic hydrogens (pKa 25) in the...Ch. 22.SE - Rank the following compounds in order of...Ch. 22.SE - Prob. 39APCh. 22.SE - Base treatment of the following , -unsaturated...Ch. 22.SE - Prob. 41APCh. 22.SE - Prob. 42APCh. 22.SE - Prob. 43APCh. 22.SE - Which, if any, of the following compounds can be...Ch. 22.SE - Prob. 45APCh. 22.SE - Prob. 46APCh. 22.SE - Prob. 47APCh. 22.SE - How might you convert geraniol into either ethyl...Ch. 22.SE - Prob. 49APCh. 22.SE - One way to determine the number of acidic...Ch. 22.SE - Prob. 51APCh. 22.SE - Prob. 52APCh. 22.SE - Prob. 53APCh. 22.SE - Prob. 54APCh. 22.SE - Prob. 55APCh. 22.SE - Prob. 56APCh. 22.SE - All attempts to isolate primary and secondary...Ch. 22.SE - How would you synthesize the following compounds...Ch. 22.SE - Prob. 59APCh. 22.SE - Prob. 60APCh. 22.SE - Prob. 61APCh. 22.SE - Prob. 62APCh. 22.SE - As far back as the 16th century, South American...Ch. 22.SE - The key step in a reported laboratory synthesis of...Ch. 22.SE - Prob. 65AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- pls helparrow_forward10) What is the [OH-] concentration of a solution with a pH of 12.0? 1.0 x 10¹² M a. b. 1.0 x 10-12 M C. 1.0 x 10² M d. 1.0 x 102M 11) In which block of the periodic table would you find the element gold? a. b. s block P block C. d block d. f blockarrow_forward5) Who was responsible for the first model of the atom that included electrons? a. Dalton b. Thomson c. Bohr d. Rutherford 6) Which of the following rate laws is a fourth-order reaction? a. r = k[X][Y]²[Z] b._r= k[X]²[Y]³ C. r = k[X][Y]² d._r= k[X][Y]²[Z]ª 7) The activation energy of a particular reaction will decrease if: a. A catalyst is used. b. Temperature is increased. c. Reactant concentration is increased. d. All of the abovearrow_forward
- pls helparrow_forwardState the reason why compound A (m.p. 99-100°C) is heated under vacuum.1. So that the sample heating temperature is not too high when heated under vacuum.2. So that the temperature is higher than the melting point of compound A.3. So that cold water is not required in the sublimator.arrow_forwardTo find the theoretical % yield of a given reaction:1. actual amount obtained once crystallized2. (actual amount obtained / theoretical amount) x 1003. maximum amount of product that can be obtained / amount of initial reactantarrow_forward
- The reason activated carbon decolorizes and purifies a product is:1. It helps dissolve the product and then recrystallize it.2. It reacts with impurities in the product and removes them.3. It retains impurities by adsorption, purifying the product.arrow_forwardThe principle of a rotary evaporator is the same as that of:1. vacuum distillation2. reflux3. fractional distillationarrow_forwardAnhydrous MgSO4 is used to:1. Form a salt with the compound dissolved in the solution2. Remove water from a solution3. Neutralize a solutionarrow_forward
- Distillation under reduced pressure or vacuum consists of:1. Achieving distillation under anhydrous conditions.2. Causing a decrease in the distillation rate.3. Decreasing the pressure to lower the boiling point of the compound to be distilled.arrow_forwardAt the end of the silica gel production process, color changes occur during drying. Explain these color changes.arrow_forwardIf CoCl2/H2O is dissolved in a mixture of H2O and concentrated HCl in a test tube, the tube is gently heated over a flame to approximately 80°C and then cooled externally. Explain the color changes that occur.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
