
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 9Q
Select the reagent needed to perform the following transformation:
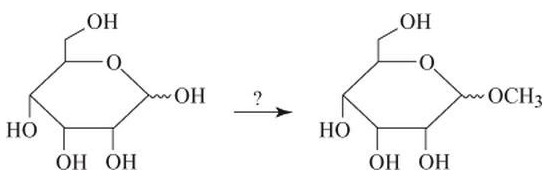
(a) CH3OH, KOH
(b) 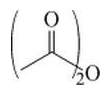
(c) (CH3)2SO4, HO−
(d) CH3OH, HCl
(e) CH3OCH3, HCl
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Finish the reactions
hand written please
Part A
Identify each alcohol as primary, secondary, or tertiary
Drag the appropriate items to their respective bins.
CH₂
H₂C-
-C-OH
HO
CH₂
Primary
Он
OH
CH₂
OH
CCH₂OH
CH₂
сн
Secondary
Tertiary
Reset Help
CH,CH₂
(CH)CHCH,OH CH,CH,CH,CCH,
CHOH
CH₂
Different types of alcohol groups
Alcohol and its reaction:
8. Combing two alcohol molecules below and completing the reaction with
Product .( Hint Reaction called etherification as ether is formed and name the
ether once you complete the reaction.
Hint.:
R-O-H+H-O-RR-O-R
Do the reaction:
CH₂OH + CH₂OH---→
+ H-O-H
9. Write the reaction of formation of alcohol from alkene by adding water:
Addition reaction also called hydration reaction as we are adding water
which occur always in presence of acid
Hint: Break the double bond and add H and OH if symmetrical then
add anywhere if unsymmetrical then follow Markovnikov rule H
should go to that double bone carbon which has more hydrogen
CH2=CH2 + H₂O-→
Complete the reaction
hand written please
Chapter 22 Solutions
Organic Chemistry
Ch. 22 - Prob. 1PPCh. 22 - Prob. 2PPCh. 22 - Prob. 3PPCh. 22 - Prob. 4PPCh. 22 - Prob. 5PPCh. 22 - Prob. 6PPCh. 22 - Prob. 7PPCh. 22 - Prob. 8PPCh. 22 - Practice Problem 22.9 What products would you...Ch. 22 - Prob. 10PP
Ch. 22 - Prob. 11PPCh. 22 - Prob. 12PPCh. 22 - Prob. 13PPCh. 22 - Prob. 14PPCh. 22 - Prob. 15PPCh. 22 - Prob. 16PPCh. 22 - Prob. 17PPCh. 22 - Prob. 18PPCh. 22 - Prob. 19PPCh. 22 - Prob. 20PCh. 22 - Prob. 21PCh. 22 - Prob. 22PCh. 22 - Prob. 23PCh. 22 - Prob. 24PCh. 22 - Prob. 25PCh. 22 - Prob. 26PCh. 22 - Prob. 27PCh. 22 - Prob. 28PCh. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Prob. 32PCh. 22 - Prob. 33PCh. 22 - Prob. 34PCh. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - Prob. 37PCh. 22 - Prob. 38PCh. 22 - Arbutin, a compound that can be isolated from the...Ch. 22 - Prob. 40PCh. 22 - Prob. 41PCh. 22 - Prob. 42PCh. 22 - Prob. 43PCh. 22 - 22.44 The following reaction sequence represents...Ch. 22 - 22.45
The NMR data for the two anomers...Ch. 22 - Shikimic acid is a key biosynthetic intermediate...Ch. 22 - Prob. 1QCh. 22 - Prob. 2QCh. 22 - Give the structural formula of the monosaccharide...Ch. 22 - Prob. 4QCh. 22 - Prob. 5QCh. 22 - Prob. 6QCh. 22 - Prob. 7QCh. 22 - Prob. 8QCh. 22 - Select the reagent needed to perform the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Distinguish between microevolution, speciation, and macroevolution.
Campbell Essential Biology (7th Edition)
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
Chemistry (7th Edition)
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
Using the forked-line, or branch diagram, method, determine the genotypic and phenotypic ratios of these trihyb...
Concepts of Genetics (12th Edition)
1. Why is it necessary to include units when reporting scientific measurements?
Introductory Chemistry (6th Edition)
DRAW IT The diagram shows a cell in meiosis. (a) Label the appropriate structures with these terms: chromosome ...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forwardIndicate the reaction products when CH3COCH2COOCH2COOC2H5 (ethyl acetoacetoacetate) reacts with 1º OH-/H2O and 2º H3O+arrow_forward
- Indicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY