
Interpretation:
The products that would be formed on salicin reacting with dilute aqueous HCl is to be determined and the mechanism of reaction for the this reaction is to be outlined.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron-rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a
舧 Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
舧 Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
舧 A carbohydrate is a
舧 Salicin is a naturally occurring glycoside and is originally found in the bark of willow trees. It is an effective analgesic for relieving pain. Its structure is given below:
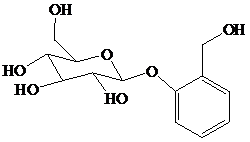
舧 Glycosides are carbohydrate acetals and can be simple or complex. These molecules are stable in basic solutions, but produce sugar and alcohol in acidic solutions.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward
- What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forward
- For benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forward
- Which of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

