
Concept explainers
(a)
Interpretation: The structure of peptide with peptide bonds, terminal amino acid and carboxyl group needs to be determined.
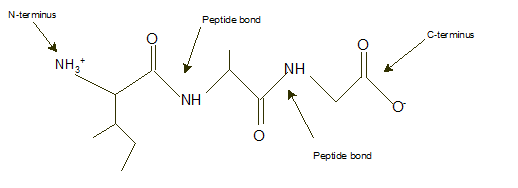
Ile-Ala-Gly
Concept Introduction: Proteins are
These amino acids involve in condensation process to form peptides and polypeptides, which further form complex protein molecules. Amino acids are the organic molecules with both
(a)
Answer to Problem 9A
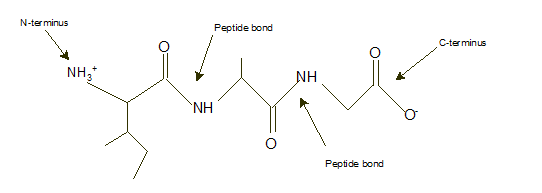
Explanation of Solution
In a tripeptide, N-terminus must be written as left side whereas the C-terminus must be right side.
(b)
Interpretation: The structure of peptide with peptide bonds , terminal amino acid and carboxyl group needs to be drawn.
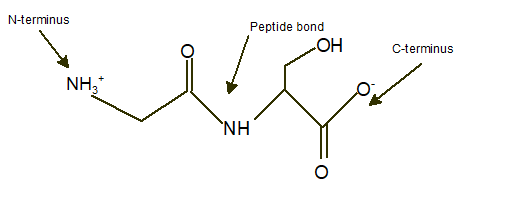
Gly-Ser
Concept Introduction: Proteins are biomolecules, which are composed of certain monomer units such as amino acid. These are carboxylic acids with one amino group bonded on alpha-C atom of the molecule.
These amino acids involve in condensation process to form peptides and polypeptides, which further form complex protein molecules. Amino acids are the organic molecules with both
(b)
Answer to Problem 9A
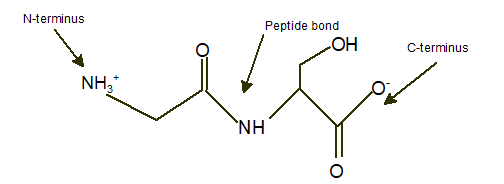
Explanation of Solution
In a dipeptide Gly-Ser, N-terminus must be written as left side whereas the C-terminus must be right side.
(c)
Interpretation: The structure of peptide with peptide bonds, terminal amino acid and carboxyl group needs to be drawn.
Ser-Gln
Concept Introduction: Proteins are biomolecules, which are composed of certain monomer units such as amino acid. These are carboxylic acids with one amino group bonded on alpha-C atom of the molecule.
These amino acids involve in condensation process to form peptides and polypeptides, which further form complex protein molecules. Amino acids are the organic molecules with both
(c)
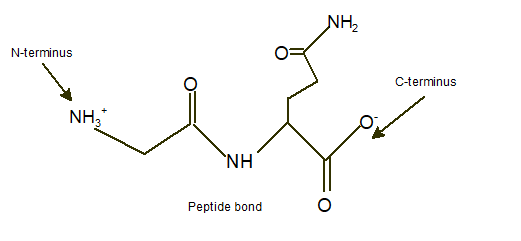
Answer to Problem 9A
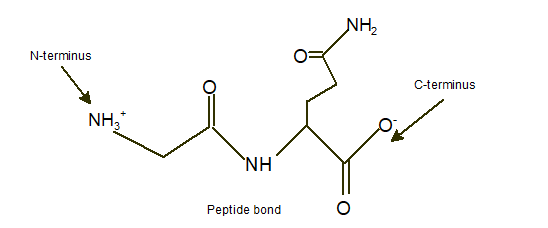
Explanation of Solution
In a dipeptide Ser-Gln, N-terminus must be written as left side whereas the C-terminus must be right side.
(d)
Interpretation: The structure of peptide with peptide bonds, terminal amino acid and carboxyl group needs to be drawn.
Cys-Asn-Gly
Concept Introduction: Proteins are biomolecules, which are composed of certain monomer units such as amino acid. These are carboxylic acids with one amino group bonded on alpha-C atom of the molecule.
These amino acids involve in condensation process to form peptides and polypeptides, which further form complex protein molecules. Amino acids are the organic molecules with both
(d)
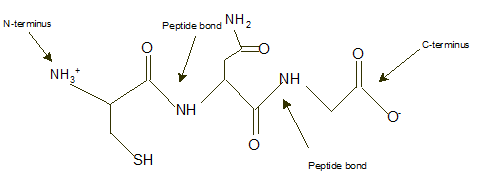
Answer to Problem 9A
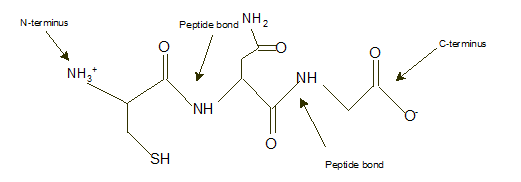
Explanation of Solution
In a tripeptide Cys-Asn-Gly, N-terminus must be written as left side whereas the C-terminus must be right side.
Chapter 21 Solutions
World of Chemistry, 3rd edition
- What would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forward
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward
- What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forwardWhat would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





