
Interpretation:
The reason for micelle formation by the soap molecules to suspend greasy dirt in a solution needs to be explained.
Concept introduction:
A micelle is a type of structure that is formed when different molecules such as soaps and detergents are mixed with water. The molecule contains fatty acid, a salt of fatty acid, phospholipids, or other similar types of compounds.
Answer to Problem 41A
The micelle is an aggregated form of surfactant in liquid to form a colloidal suspension.
The hydrophobic part of the soap is towards the dirt part and the hydrophilic part projects outside in the bristle’s manner.
Explanation of Solution
A micelle is a composition of surfactant molecules scattered in a liquid that is a colloid. A complete micelle form in an aqueous solution having two phases one is hydrophilic and the other one is hydrophobic. The hydrophilic head regions in contact withthe solvent, and attached hydrophobic single tail regions in the central side of the micelle.
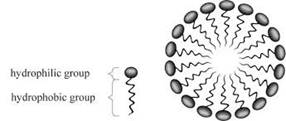
The greasy dirt is present at the center of the micelle and surrounded by the hydrophobic part. It combines with the dirt and hydrophilic part dissolved in the water to rinse out the dirt.
The molecules of soap arrange in a rounded shape in micelle formation and it covers the dirt at the center part of the cluster. The formed micelles are remain suspended in the water resulting a colloidal solution. The hydrophobic ends attached to dirt and washout with water.
Chapter 21 Solutions
World of Chemistry, 3rd edition
- First I wanted to see if you would mind checking my graphs behind me. (They haven't been coming out right)? Second, could you help me explain if the rate of reaction is proportional to iodide and persulfate of each graph. I highlighted my answer and understanding but I'm not sure if I'm on the right track. Thank you in advance.arrow_forwardThe heat of combustion for ethane, C2H6C2H6 , is 47.8 kJ/g. How much heat is produced if 1.65 moles of ethane undergo complete combustion?arrow_forwardReview of this week's reaction: H2NCN (cyanamide) + CH3NHCH2COOH (sarcosine) + NaCl, NH4OH, H2O ----> H2NC(=NH)N(CH3)CH2COOH (creatine) Q7. Draw by hand the reaction of creatine synthesis listed above using line structures without showing the Cs and some of the Hs, but include the lone pairs of electrons wherever they apply. (4 pts) Q8. Considering the Zwitterion form of an amino acid, draw the Zwitterion form of Creatine. (2 pts) Q9. Explain with drawing why the C—N bond shown in creatine structure below can or cannot rotate. (3 pts)arrow_forward
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forwardPlease help me answer a. Please and thank you I advance.arrow_forwardDraw both of the chair flips for both the cis and trans isomers for the following compounds: 1,4-diethylcyclohexane 1-methyl-3-secbutylcyclohexanearrow_forward
- Ppplllleeeaaasssseeee hellppp wiithhh thisss physical chemistryyyyy I talked like this because AI is very annoyingarrow_forwardFor this question, if the product is racemic, input both enantiomers in the same Marvin editor. A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a racemic product. Input 1 for Cl, in the cold and dark Input 2 for Oy followed by H₂O, Zn Input 3 for D₂ with metal catalyst Input 4 for H₂ with metal catalyst B) Draw the skeletal structure of the major organic product made from the reagent in part A Marvin JS Help Edit drawing C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with peroxyacetic acid. Marvin 35 Helparrow_forwardMichael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forward
- Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





