
Concept explainers
(a)
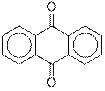
Interpretation: The given compound anthraquinone needs to be identified as an
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 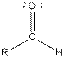 |
| Ketone | 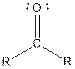 |
| Carboxylic acid | 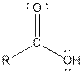 |
| Ester | 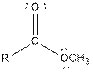 |
| Amine | 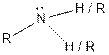 |
(a)
Answer to Problem 37E
Anthraquinone contains a ketone functional group.
Explanation of Solution
From the table, the general structure for ketone is as follows:
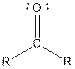
The given compound contains a ketone functional group shown as:
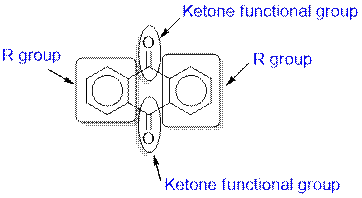
(b)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
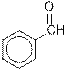
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 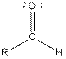 |
| Ketone | 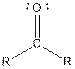 |
| Carboxylic acid | 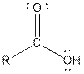 |
| Ester | 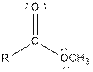 |
| Amine | 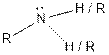 |
(b)
Answer to Problem 37E
The given organic compound contains an aldehyde functional group.
Explanation of Solution
From the table, the general structure for an aldehyde is as follows:
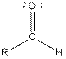
The given compound contains an aldehyde functional group shown as:

(c)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
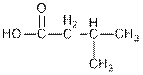
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 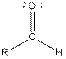 |
| Ketone | 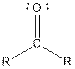 |
| Carboxylic acid | 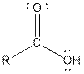 |
| Ester | 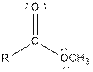 |
| Amine | 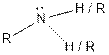 |
(c)
Answer to Problem 37E
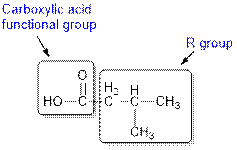
The given organic compound contains a carboxylic acid functional group.
Explanation of Solution
From the table, the general structure for carboxylic acid functional group is as follows:
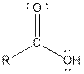
The given compound contains a carboxylic acid functional group shown as:
(d)
Interpretation: The given compounds needs to be identified as an aldehyde, ester, carboxylic acid, ketone or amine.
Concept Introduction: Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 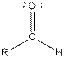 |
| Ketone | 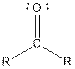 |
| Carboxylic acid | 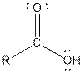 |
| Ester | 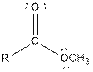 |
| Amine | 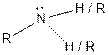 |
(d)
Answer to Problem 37E
The given organic compound contains an amine functional group.
Explanation of Solution
From the table, the general structure for amine functional group is as follows:
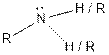
The given compound contains a carboxylic acid functional group shown as:
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forwardQuestion 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forward
- Identify the missing starting materials/ reagents/ products in the following reactions. Show the stereochemistry clearly in the structures, if any. If there is a major product, draw the structures of the major product with stereochemistry clearly indicated where applicable. Show only the diastereomers (you do not have to draw the pairs of enantiomers). If you believe that multiple products are formed in approximately equal amounts (hence neither is the major product), draw the structures of the products, and show the detailed mechanism of these reactions to justify the formation of the multiple products. If you believe no product is formed, explain why briefly. (6 mark for each, except f and g, which are 10 mark each)arrow_forward3. What starting material would you use to synthesize 3-hydroxypentanoic acid using a NaBH4 reduction?arrow_forward1. Give stereochemical (Fischer projection) formulas for all (but no extras) the stereoisomers that could theoretically form during the reduction of a. the carbonyl group of 2-methyl-3--pentanone b. both carbonyl groups of 2,4-pentanedione (careful!) 2. Predict the products of the reduction of O=CCH2CH2CH2C=O with a. LiAlH4 b. NaBH4 CH3 OHarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





