
Concept explainers
(a)
Interpretation:
The name of the following alkene is to be determined.
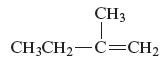
Concept Introduction :
The
(a)
Answer to Problem 163AE
The name of the given alkene is 2 − methyl − 1 butene.
Explanation of Solution
The structure of the compound is given as follows:
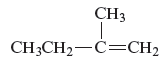
From the above structure, it can be seen that there are four carbon atoms present in the principle chain of the compound and the atoms are bonded with double bond. Hence, the compound is known as alkene. The double bond of the structure is present at the position one that is the lowest possible number. The substituent methyl of the structure is present at second carbon (C2). Therefore, the name of the compound is 2 − methyl − 1 butene. The representation of the carbon position in a structure is given below −
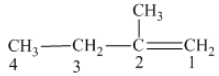
(b)
Interpretation:
The name of the following alkene is to be determined.
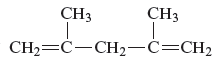
Concept Introduction :
The unsaturated hydrocarbon is a group of hydrocarbons and also known as alkenes or alkynes. They are unsaturated because of the presence of triple or double bonds. Alkynes or hydrocarbons consist of at least one triple bond while alkenes consist of at least one carbon-carbon double bond in the main chain.
(b)
Answer to Problem 163AE
The name of the given alkenes is 2, 4 − dimethyl − 1, 4 − pentadiene.
Explanation of Solution
The structure of the given compound is −
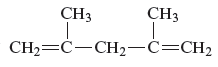
From the above structure, it can be seen that there are five carbon atoms are present in the principle chain of the compound and the atoms are bonded with two double bonds. Hence, the compound is known as alkene. The double of the structure is present at the position one and position four and these are the lowest possible numbers. The methyl substituent of the structure is present at second carbon (C2) and third carbon (C3). Therefore, the name of the compound is 2, 4 − dimethyl − 1, 4 − pentadiene. The representation of the carbon position in a structure is given below −
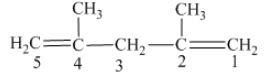
(c)
Interpretation:
The name of the following alkene is to be determined
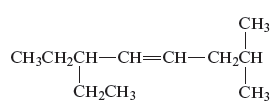
Concept Introduction :
The unsaturated hydrocarbon is a group of hydrocarbons and also known as alkenes or alkynes. They are unsaturated because of the presence of triple or double bonds. Alkynes or hydrocarbons consist of at least one triple bond while alkenes consist of at least one carbon-carbon double bond in the main chain.
(c)
Answer to Problem 163AE
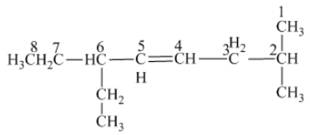
The name of the given alkene is 6 − ethyl − 2 − methyl − 4 octene.
Explanation of Solution
The structure of the given compound is −
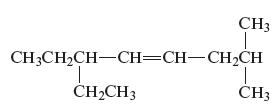
From the above structure, it can be seen that there are eight carbon atoms are present in the principle chain of the compound and there is a double bond in the main chain. Hence, the compound is known as alkene. The substituent ethyl is present at the sixth carbon (C6) atom and the substituent methyl is present at the second carbon atom (C2). Therefore, the name of the compound is 6 − ethyl − 2 − methyl − 4 octene. The representation of the carbon position in a structure is given below −
(d)
Interpretation:
The name of the following alkyne is to be determined.
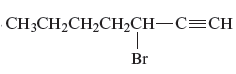
Concept Introduction :
The unsaturated hydrocarbon is a group of hydrocarbons and also known as alkenes or alkynes. They are unsaturated because of the presence of triple or double bonds. Alkynes or hydrocarbons consist of at least one triple bond while alkenes consist of at least one carbon-carbon double bond in the main chain.
(d)
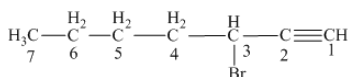
Answer to Problem 163AE
The name of the given alkyne is 3 − bromo-1-heptyne.
Explanation of Solution
The structure of the given compound is −
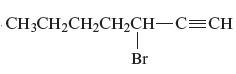
From the above structure, it can be seen that there are seven carbon atoms present in the principle chain of the compound and the atoms are bonded with triple bonds. Hence, the compound is known as alkyne. The triple bond of the structure is present at position one that is lowest possible number. The substituent bromine of the structure is present at third carbon (C3) position. Therefore, the name of the compound is 3 − bromo − 1 − heptyne. The representation of the carbon position in a structure is given below −
(e)
Interpretation:
The name of the following alkyne is to be determined
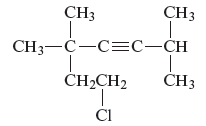
Concept Introduction :
The unsaturated hydrocarbon is a group of hydrocarbons and also known as alkenes or alkynes. They are unsaturated because of the presence of triple or double bonds. Alkynes or hydrocarbons consist of at least one triple bond while alkenes consist of at least one carbon-carbon double bond in the main chain.
(e)
Answer to Problem 163AE
The name of the given alkyne is 7 − chloro − 2, 5,5 − trimethyl − 3 − heptyne.
Explanation of Solution
The structure of the given compound is −
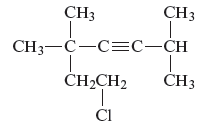
From the above structure, it can be seen that there are six carbon atoms are present in the principle chain of the compound and the atoms are bonded with triple bonds. Hence, the compound is known as alkynes. The triple bond of the structure is present at position three that is lowest possible number. The two methyl substituents of the structure are present at fifth carbon (C5) position and one methyl substituent is present at second carbon (C2) position. The other chlorine substituent is present at seventh carbon position (C7). Therefore, the name of the compound is 7 − chloro − 2, 5,5 − trimethyl − 3 − heptyne. The representation of the carbon position in a structure is given below −
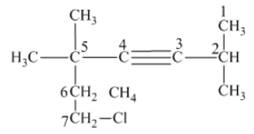
(f)
Interpretation:
The name of the following alkyne is to be determined
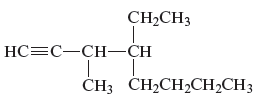
Concept Introduction:
The unsaturated hydrocarbon is a group of hydrocarbons and also known as alkenes or alkynes. This structure is formed because of the presence of triple or double bonds. Alkynes or hydrocarbons consists at least triple bond while alkynes consist at least one carbon-carbon double bon in the main chain.
(f)
Answer to Problem 163AE
The name of the given alkyne is 4 − ethyl − 3 − methyl − 1 − octyne.
Explanation of Solution
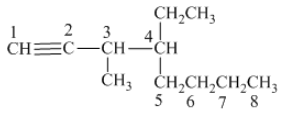
The structure of the given compound is −
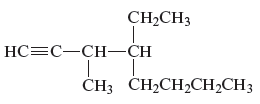
From the above structure, it can be seen that there are eight carbon atoms are present in the principle chain of the compound and the atoms are bonded with triple bonds. Hence, the compound is known as alkynes. The triple bond of the structure is present at position one that is lowest possible number. The methyl substituent of the structure is present at third carbon (C3) position and one ethyl substituent is present at fourth carbon (C4) position. Therefore, the name of the compound is 4 − ethyl − 3 − methyl − 1 − octyne. The representation of the carbon position in a structure is given below −
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) | Bakelite like polymer Using: Resorcinol + NaOH + Formalinarrow_forwardQuestion 19 0/2 pts 3 Details You have a mixture of sodium chloride (NaCl) and potassium chloride (KCl) dissolved in water and want to separate out the Cl- ions by precipitating them out using silver ions (Ag+). The chemical equation for the net ionic reaction of NaCl and KCl with silver nitrate, AgNO3, is shown below. Ag+(aq) + Cl(aq) → AgCl(s) The total mass of the NaCl/KCl mixture is 1.299 g. Adding 50.42 mL of 0.381 M solution precipitates out all of the Cl-. What are the masses of NaCl and KCl in the mixture? Atomic masses: g: Mass of NaCl g: Mass of KCL Ag = 107.868 g mol- 1 Cl = 35.453 g mol- 1 K = 39.098 g mol- N = 14.007 g mol−1 Na = 22.99 g mol−1 0 = 15.999 g mol 1 Question Help: ✓ Message instructor Submit Questionarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerolarrow_forward
- Identify the missing starting materials/ reagents/ products in the following reactions. Show the stereochemistry clearly in the structures, if any. If there is a major product, draw the structures of the major product with stereochemistry clearly indicated where applicable. Show only the diastereomers (you do not have to draw the pairs of enantiomers). If you believe that multiple products are formed in approximately equal amounts (hence neither is the major product), draw the structures of the products, and show the detailed mechanism of these reactions to justify the formation of the multiple products. If you believe no product is formed, explain why briefly. (6 mark for each, except f and g, which are 10 mark each)arrow_forward3. What starting material would you use to synthesize 3-hydroxypentanoic acid using a NaBH4 reduction?arrow_forward1. Give stereochemical (Fischer projection) formulas for all (but no extras) the stereoisomers that could theoretically form during the reduction of a. the carbonyl group of 2-methyl-3--pentanone b. both carbonyl groups of 2,4-pentanedione (careful!) 2. Predict the products of the reduction of O=CCH2CH2CH2C=O with a. LiAlH4 b. NaBH4 CH3 OHarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





