
Concept explainers
(a)
Interpretation:
The structure of
Concept introduction:
Answer to Problem 21.1P
The structure of
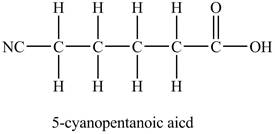
Explanation of Solution
In
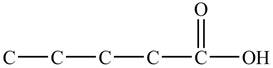
Figure 1
At the fifth carbon, there is a cyano group
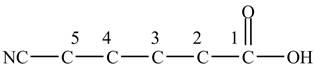
Figure 2
The above skeleton is completed by adding hydrogens as per the tetravalency of carbon as shown below.
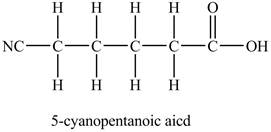
Figure 3
The structure of
(b)
Interpretation:
The structure of isopropyl valerate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of isopropyl valerate is shown below.
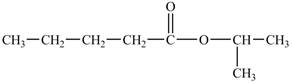
Explanation of Solution
The structure of isopropyl valerate consists of eight carbons, sixteen hydrogen atoms and two oxygen atoms. Isopropyl valerate is a common name of isopropyl pentanoate. It is a five carbon carboxylic acid. The structure of pentanoic acid is shown below.
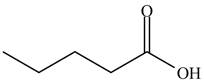
Figure 4
The hydrogen of carboxylic group is replaced by an isopropyl group to form an ester. The structure of isopropyl valerate is shown below.
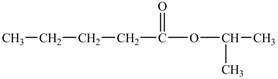
Figure 5
The structure of isopropyl valerate is shown in Figure 5.
(c)
Interpretation:
The structure of ethyl methyl malonate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of ethyl methyl malonate is shown below.
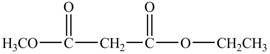
Explanation of Solution
The structure of ethyl methyl malonate consists of a eight carbons, fourteen hydrogen and four oxygen. Ethyl methyl malonate consists of propane dioic acid as parent chain.
The structure of propane dioic acid is shown below.
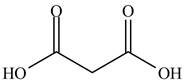
Figure 6
In the above structure, at first position hydrogen is substituted by a methyl group and at the last carboxyl group an ethyl group replaces the hydrogen of the carboxyl group. The structure of ethyl methyl malonate is shown below.
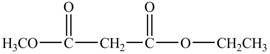
Figure 7
The structure of ethyl methyl malonate is shown in Figure 6.
(d)
Interpretation:
The structure of cyclohexyl acetate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of cyclohexyl acetate is shown below.
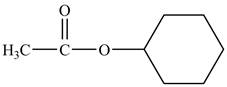
Explanation of Solution
Cyclohexyl acetate consists of a cyclohexane ring and an acetate group. The acetate group is given by the formula
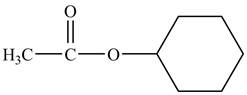
Figure 8
The structure of cyclohexyl acetate is shown in Figure 8
(e)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
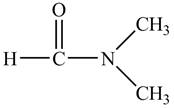
Explanation of Solution
The structure of
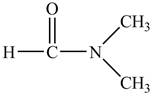
Figure 9
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
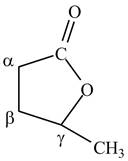
Explanation of Solution
Lactones are cyclic esters. The structure of
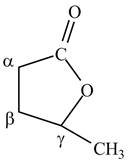
Figure 10
The structure of
(g)
Interpretation:
The structure of glutarimide is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of glutarimide is shown below.
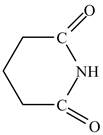
Explanation of Solution
The structure of glutarimide consists of a piperidine ring. There are two carbonyl group present adjacent to the nitrogen group. The structure of a piperidine ring is shown below.
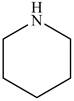
Figure 11
Therefore, the structure of glutarimide is shown below.
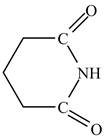
Figure 12
The structure of glutarimide is shown in Figure 12.
(h)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
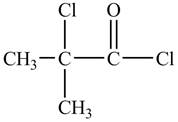
Explanation of Solution
There is carboxyl group present in
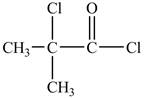
Figure 13
The structure of
(i)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
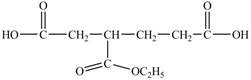
Explanation of Solution
In
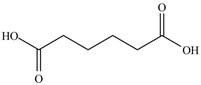
Figure 14
In the above structure there is a ethoxy carbonyl group at the third position. Therefore, the structure of
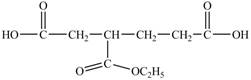
Figure 15
The structure of
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry Study Guide and Solutions
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward
- Can I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

