
Concept explainers
Label each compound as a hydrolyzable or nonhydrolyzable lipid.
- prostaglandin
- phosphoacylglycerol
- triacylglycerol
- lecithin
- leukotriene
- cholesterol
- vitamin A
(a)
Interpretation:
Prostaglandin should be labeled as Hydrolyzable or non-hydrolyzable lipid.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are subgroups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Nonhydrolyzable
Explanation of Solution
Prostaglandin is a 20 carbon unsaturated carboxylic acid which contains a five-membered ring. A typical structure for prostaglandin is,
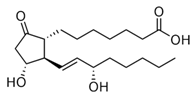
Prostaglandin does not contain an ester group. So, prostaglandin is nonhydrolyzable lipid.
(b)
Interpretation:
Triacylglycerol should be labeled as Hydrolyzable or non-hydrolyzable.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are subgroups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Hydrolyzable
Explanation of Solution
Triacylglycerol is produced by reacting a glycerol molecule with three molecules of fatty acids by an ester linkage.
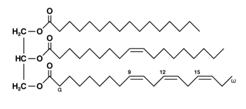
Triacylglycerol has three ester linkages. So, triacylglycerol is a hydrolyzable lipid.
(c)
Interpretation:
Leukotriene should be labeled as Hydrolyzable or non-hydrolyzable.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are subgroups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Nonhydrolyzable
Explanation of Solution
Leukotrienes belong to the family of eicosanoids. Leukotrienes are synthesized by arachidonic acid. A typical structure for leukotriene is,
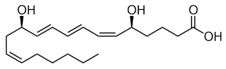
Leukotrienes have no ester linkages. So, leukotrienes are nonhydrolyzable lipids.
(d)
Interpretation:
Vitamin A should be labeled as hydrolyzable or non-hydrolyzable.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are subgroups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Nonhydrolyzable
Explanation of Solution
Vitamin A is a group of several unsaturated fatty acids. It includes retinol which has an alcohol functional group, retinal which is an aldehyde and retinoic acid which is a carboxylic acid.
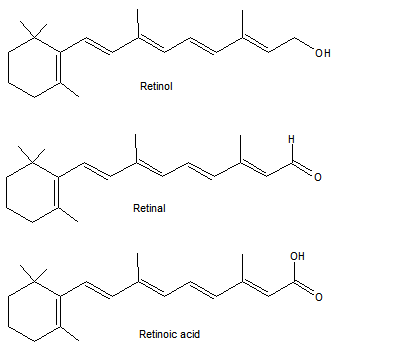
No structure has an ester linkage. So vitamin A is nonhydrolyzable lipid.
(e)
Interpretation:
Phoshoacylglycerol should be labeled as hydrolyzable or non-hydrolyzable.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are subgroups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Hydrolyzable
Explanation of Solution
Phosphoacylglycerols are esterified glycerol in which, two hydroxyl groups of glycerol are esterified with fatty acids and one hydroxyl is esterified with phosphoric acid.
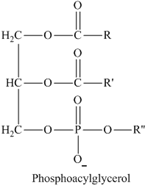
Since phosphoacylglycerol has ester linkages, it is a hydrolyzable lipid.
(f)
Interpretation:
Lecithin should be labeled as hydrolyzable or non-hydrolyzable.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are subgroups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Hydrolyzable
Explanation of Solution
Lecithins are mixtures of glycerophospholipids. As glycerophospholipids are esterified form of glycerol with two fatty acids and phosphoric acid. Lecithin contains ester linkages. So, lecithin is a hydrolyzable lipid. The structure of phosphatidylcholine which is a type of phospholipid in lecithin is given below.
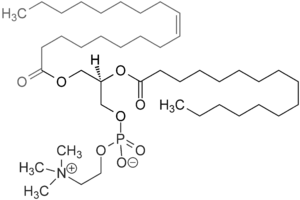
(g)
Interpretation:
Cholesterol should be labeled as hydrolyzable or non-hydrolyzable.
Concept Introduction:
Lipids can be grouped as hydrolyzable and nonhydrolyzable. Hydrolyzable lipids can be broken down into smaller molecules by reacting with water. Hydrolyzable lipids usually contain an ester functional group which is hydrolyzable. There are three subgroups of hydrolyzable lipids as waxes, triacylglycerols and phospholipids. Nonhydrolyzable lipids cannot be broken down into smaller molecules by hydrolysis with water. There are sub groups of nonhydrolyzable lipids as steroids, fat-soluble vitamins and eicosanoids.
Answer to Problem 19.29P
Nonhydrolyzable
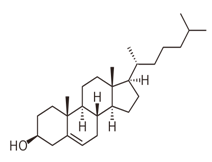
Explanation of Solution
Cholesterol is a type of sterol and has no ester linkages. So, cholesterol is nonhydrolyzable lipid.
Want to see more full solutions like this?
Chapter 19 Solutions
General, Organic, & Biological Chemistry
- Draw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forwardDraw the chemical structure [OR IUPAC name] of the following: a- m-chloromethoxybenzene b.arrow_forward
- Show by chemical equation the reaction of [HCN] and [CH3MgBr] with any alarrow_forwardGive the chemical equation for the preparation of: -Any aldehyde -Any keytonearrow_forward+ C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward
- → Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- Identifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardAssign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



