
Interpretation:
The two types of bonding which will occur in the bonding of the enzyme-substrate complex should be given with dotted lines.
Concept Introduction:
Enzyme:
- It is a protein or a molecule which can act as a catalyst for a biological reaction.
- Does not affect the equilibrium point of the reaction.
- Active site of the enzyme is the region where the reaction takes place.
- Enzyme’s activity can be specific which means the activity is limited to a certain substrate and a certain type of reaction and it is referred to as specificity of the enzyme.
Hydrogen bonding: It is an unusual strong intermolecular force occurs between a hydrogen atom and an electronegative atom like nitrogen, oxygen or fluorine. It occurs in both water and ammonia
Answer to Problem 19.24UKC
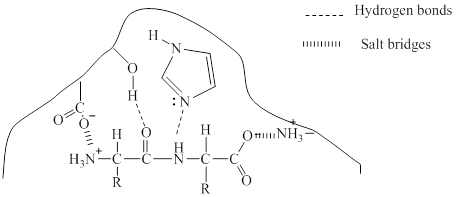
Explanation of Solution
Enzymes are three dimensional molecule with a catalytic site into which the substrate can fit. The enzymes are said to be so specific in their action because the activity is limited to a certain substrate and a certain type of reaction and it is referred to as specificity of the enzyme. It can be said that few molecules have the appropriate shape and
Given in the diagram a dipeptidase enzyme and the substrate to form the enzyme-substrate complex. Different types of bonding may occur between the enzyme and the substrate to form the enzyme-substrate complex.
Hydrogen bonding is the attraction between a hydrogen atom and an electronegative atom and it can be considered as a special type of dipole-dipole interaction. The oxygen atom in the substrate can make hydrogen bond with hydrogen in the enzyme.
Salt bridges are formed between oppositely charged residues that are nearby to experience electrostatic attraction. Here, salt bridge is formed between the anionic carboxylate ion
The bonding is shown below:
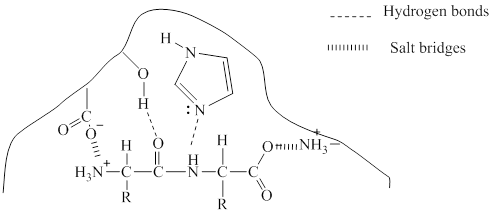
The two types of bonding which will occur in the bonding of the enzyme-substrate complex is given with dotted lines.
Want to see more full solutions like this?
Chapter 19 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- Draw the reaction between sphingosine and arachidonic acid. Draw out the full structures.arrow_forwardDraw both cis and trans oleic acid. Explain why cis-oleic acid has a melting point of 13.4°C and trans-oleic acid has a melting point of 44.5°C.arrow_forwardDraw the full structure of the mixed triacylglycerol formed by the reaction of glycerol and the fatty acids arachidic, lauric and trans-palmitoleic. Draw the line structure.arrow_forward
- Draw out the structure for lycopene and label each isoprene unit. "Where is lycopene found in nature and what health benefits does it provide?arrow_forwardWhat does it mean to be an essential fatty acid? What are the essential fatty acids?arrow_forwardCompare and contrast primary and secondary active transport mechanisms in terms of energy utilisation and efficiency. Provide examples of each and discuss their physiological significance in maintaining ionic balance and nutrient uptake. Rubric Understanding the key concepts (clearly and accurately explains primary and secondary active transport mechanisms, showing a deep understanding of their roles) Energy utilisation analysis ( thoroughly compares energy utilisation in primary and secondary transport with specific and relevant examples Efficiency discussion Use of examples (provides relevant and accurate examples (e.g sodium potassium pump, SGLT1) with clear links to physiological significance. Clarity and structure (presents ideas logically and cohesively with clear organisation and smooth transition between sections)arrow_forward
- 9. Which one of the compounds below is the major organic product obtained from the following reaction sequence, starting with ethyl acetoacetate? 요요. 1. NaOCH2CH3 CH3CH2OH 1. NaOH, H₂O 2. H3O+ 3. A OCH2CH3 2. ethyl acetoacetate ii A 3. H3O+ OH B C D Earrow_forward7. Only one of the following ketones cannot be made via an acetoacetic ester synthesis. Which one is it? Ph کہ A B C D Earrow_forward2. Which one is the major organic product obtained from the following reaction sequence? HO A OH 1. NaOEt, EtOH 1. LiAlH4 EtO OEt 2. H3O+ 2. H3O+ OH B OH OH C -OH HO -OH OH D E .CO₂Etarrow_forward
- what is a protein that contains a b-sheet and how does the secondary structure contributes to the overall function of the protein.arrow_forwarddraw and annotate a b-sheet and lable the hydrogen bonding. what is an example that contains the b-sheet and how the secondary structure contributes to the overall function of your example protein.arrow_forwardFour distinct classes of interactions (inter and intramolecular forces) contribute to a protein's tertiary and quaternary structures. Name the interaction then describe the amino acids that can form this type of interaction. Draw and annotate a diagram of the interaction between two amino acids.arrow_forward
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning





