
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.4, Problem 5P
Which of the following amino acid side chains can help remove a proton from the α-Carbon of an
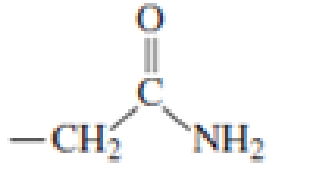
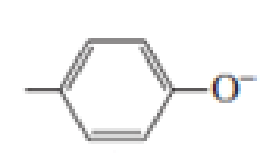
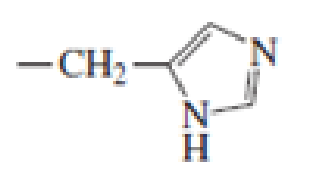
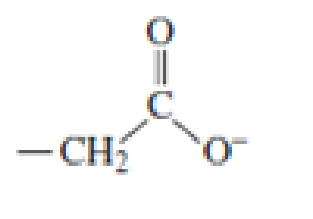
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show work. Don't give Ai generated solution
5. Please draw in the blanks the missing transition states and the correlated products. Explicitly
display relevant absolute stereochemical configuration.
MeOH
I
OMe
H
Endo transition state,
dienophile approaching from the bottom of diene
+
H
ཎྞཾ ཌཱརཱ༔,_o
OMe
H
H
OMe
Endo transition state,
dienophile approaching from the top of diene or
from the bottom but horizontally flipped (draw one)
+
Exo transition state,
dienophile approaching from the top of diene or
from the bottom but horizontally flipped (draw one)
Exo transition state,
dienophile approaching from the top of diene or
from the bottom but horizontally flipped (draw one)
MeO H
H
MeO H
MeO H
MeO H
H
H
H
(1)
H
C.
C
C
.H
(2)
(3)
Cl
H
The ideal value for bond angle (1) is (Choose one)
and the ideal value for bond angle (3) is (Choose one)
degrees, the value for bond angle (2) is (Choose one) degrees,
degrees.
Chapter 18 Solutions
Essential Organic Chemistry, Global Edition
Ch. 18.1 - Prob. 1PCh. 18.2 - If H218O were used to hydrolyze lysozyme, which...Ch. 18.3 - Which of the following amino acid side chains can...Ch. 18.3 - Arginine and lysine side chains fit into trypsins...Ch. 18.4 - Which of the following amino acid side chains can...Ch. 18.4 - Prob. 6PCh. 18.5 - Prob. 7PCh. 18.5 - Draw the mechanism for the hydroxide-ion-catalyzed...Ch. 18.5 - What advantage does the enzyme gain by forming an...Ch. 18.7 - Prob. 10P
Ch. 18.7 - Prob. 11PCh. 18.8 - How many conjugated double bonds are there in a....Ch. 18.8 - Instead of adding to the 4a-position and...Ch. 18.8 - In succinate dehydrogenase, FAD is covalently...Ch. 18.8 - Prob. 15PCh. 18.9 - Acetolactate synthase is another TPP-requiring...Ch. 18.9 - Acetolactate synthase can also transfer the acyl...Ch. 18.9 - Prob. 18PCh. 18.9 - Prob. 19PCh. 18.10 - Prob. 21PCh. 18.11 - Prob. 23PCh. 18.11 - Which compound is more easily decarboxylated?Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.11 - Explain why the ability of PLP to catalyze an...Ch. 18.12 - What groups are interchanged in the following...Ch. 18.13 - Why is the coenzyme called tetrahydrofolate?Ch. 18.13 - What amino acid is formed by the following...Ch. 18.13 - How do the structures of tetrahydrofolate and...Ch. 18.13 - What is the source of the methyl group in...Ch. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - From what vitamins are the following coenzymes...Ch. 18 - Prob. 35PCh. 18 - For each of the following reaction, name both the...Ch. 18 - Explain why serine proteases do not catalyze...Ch. 18 - Prob. 38PCh. 18 - For each of the following enzyme catalyzed...Ch. 18 - Trisephosphate isomerase (TIM) catalyzes the...Ch. 18 - Prob. 41PCh. 18 - What acyl groups have we seen transferred by...Ch. 18 - When UMP is dissolved in T2O, exchange of T for H...Ch. 18 - Prob. 44PCh. 18 - When transaminated, the three branched-chain amino...Ch. 18 - Aldolase shows no activity if it is incubated with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show work.....don't give Ai generated solutionarrow_forwardShow work. Don't give Ai generated solutionarrow_forward10. Complete the following halogenation reactions for alkanes. Draw the structures of one of the many possible products for each reaction. Name the reactant and product. a) CH₂- CH-CH2-CH3 + Br₂ CH₂ UV UV b) + Cl2 c) CH3-CH₂ CHICHCHICH-CH CH₂-CH₂ + F2 UVarrow_forward
- Which of the following processes involves the largest photon energy? Group of answer choices Electron promotion from n=2 to n=5 Electron relaxing from n=4 to n=3 Ionization of an electron from n=2 Ionization of an electron from n=4arrow_forwardWhich of the following compounds does not match atomic ratio expectations in Mendeleev's 1872 periodic table? Group of answer choices NO2 Al2O3 SO3 CaOarrow_forwardNeed help with 14 and 15. 14. bromobenzene + (CHs),CuLi + THF / -78° followed by water quench is a. toluene else!! b. xylene c. cumene d. styrene e. something 15. When cumene + H,SO, / Na,Cr, 0,/water are mixed (refluxed) what is produced? a. 2-phenylpropanol phenol e. styrene b. benzoic acid c. no reaction!arrow_forward
- Which of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.) Group of answer choices px dxz dx2-y2 py dxy sarrow_forwardWhich of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices Li-Na-K Al-Ga-In Cr-Mo-W K-Rb-Csarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forwardGive the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY