
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 18.29P
18-29 Complete the equations for these acid—base reactions.
(a) 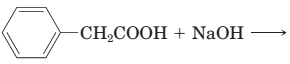
(b) 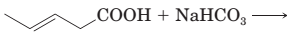
(c) 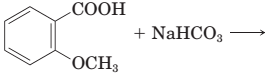
(d) 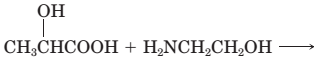
(e) 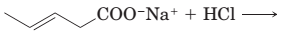
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of the following organic reaction:
O O
+
A
?
Some important notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
Explanation Check
Click and drag to start drawing a structure.
eserved. Terms of Use | Privacy Center
>
(EXM 2, PRBLM 3) Here is this problem, can you explain it to me and show how its done. Thank you I need to see the work for like prbl solving.
can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products
Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below
Chapter 18 Solutions
Introduction to General, Organic and Biochemistry
Ch. 18.2 - Prob. 18.1PCh. 18.5 - Prob. 18.2PCh. 18.5 - Prob. 18.3PCh. 18 - 18-4 Answer true or false. (a) The functional...Ch. 18 - Prob. 18.5PCh. 18 - 18-6 Name and draw structural formulas for the...Ch. 18 - 18-7 Write the IUPAC name for each carboxylic...Ch. 18 - 18-8 Write the IUPAC name for each carboxylic...Ch. 18 - Prob. 18.9PCh. 18 - Prob. 18.10P
Ch. 18 - Prob. 18.11PCh. 18 - Prob. 18.12PCh. 18 - Prob. 18.13PCh. 18 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 18 - 18-15 Draw a structural formula for the dimer...Ch. 18 - 18-16 Propanedioic (malonic) acid forms an...Ch. 18 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 18 - 18-18 Propanoic acid and methyl acetate are...Ch. 18 - 18-19 The following compounds have approximately...Ch. 18 - Prob. 18.20PCh. 18 - Prob. 18.21PCh. 18 - Prob. 18.22PCh. 18 - 18-23 Characterize the structural features...Ch. 18 - Prob. 18.24PCh. 18 - Prob. 18.25PCh. 18 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 18 - Prob. 18.27PCh. 18 - 18-28 Arrange these compounds in order of...Ch. 18 - 18-29 Complete the equations for these acid—base...Ch. 18 - 18-30 Complete the equations for these acid-base...Ch. 18 - 18-31 Formic acid is one of the components...Ch. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 18 - Prob. 18.39PCh. 18 - Prob. 18.40PCh. 18 - 18-41 Complete these examples of Fischer...Ch. 18 - Prob. 18.42PCh. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 18 - 18-47 Methylparaben and propylparaben are used as...Ch. 18 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Prob. 18.51PCh. 18 - Prob. 18.52PCh. 18 - Prob. 18.53PCh. 18 - Prob. 18.54PCh. 18 - Prob. 18.55P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forwardDraw the mechanism of friedel-crafts acylation using acetyl chloride of m-Xylenearrow_forward
- Don't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardDraw the friedel-crafts acylation mechanism of m-Xylenearrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward1. Base on this experimental results, how do you know that the product which you are turning in is methyl 3-nitrobenzoate(meta substituted product ) rather than either of the other two products? 2. What observation suggests that at least a small amount of one or both of the other two isomers are in the mother liquor?arrow_forwardExplain Huckel's rule.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Molecular spectroscopy; Author: Vidya-mitra;https://www.youtube.com/watch?v=G6HjLIWvCQo;License: Standard YouTube License, CC-BY