
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 57P
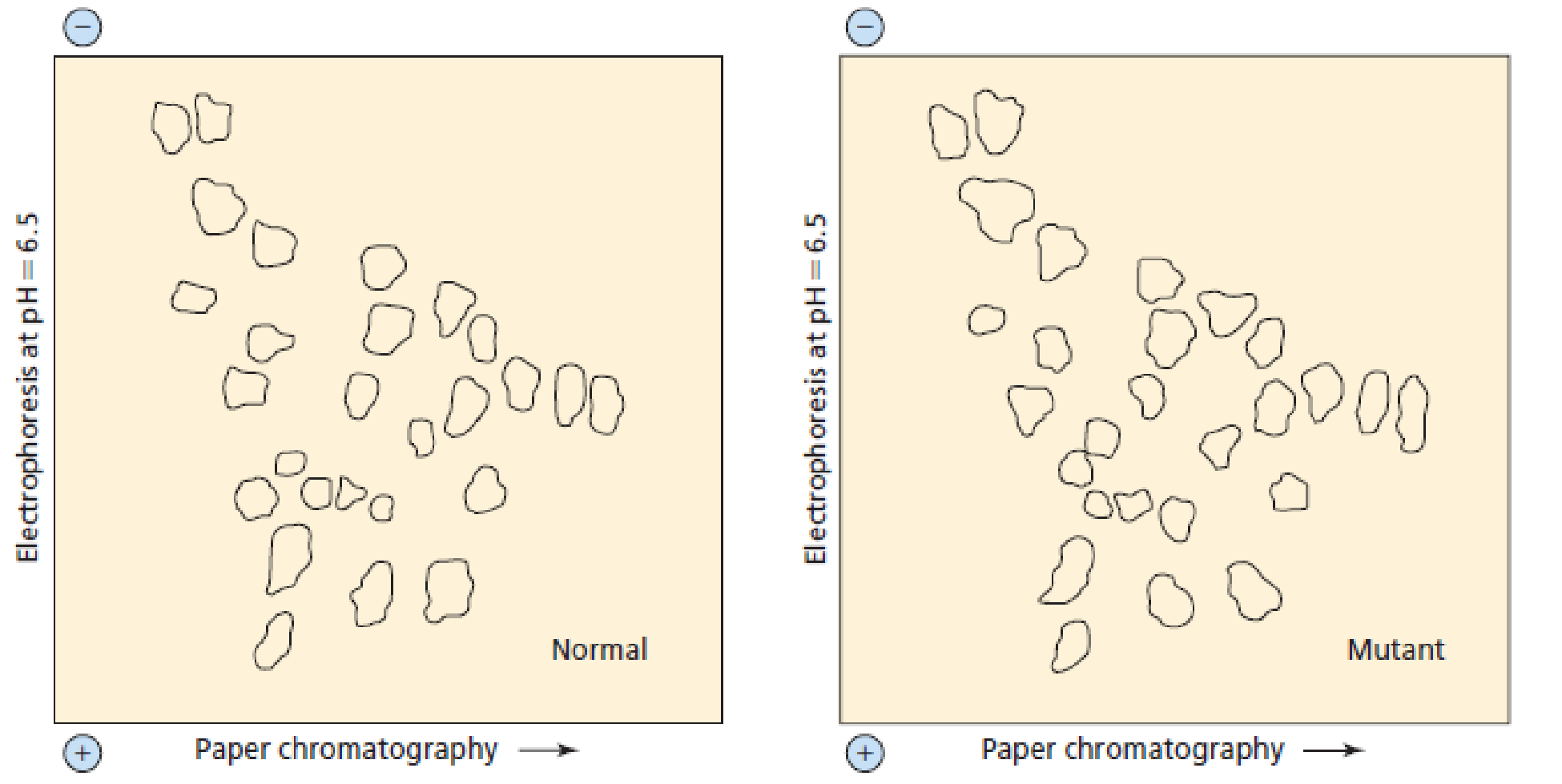
A normal polypeptide and a mutant of the polypeptide were hydrolyzed by an endopeptidase under the same conditions. The normal and mutant polypeptide differ by one amino acid. The fingerprints of the peptides obtained from the two polypeptides are shown here. What kind of amino acid substitution occurred as a result of the mutation? (That is, is the substituted amino acid more or less polar than the original amino acid? Is its pI lower or higher?) (Hint: Photocopy the fingerprints, cut them out, and overlay them.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Fill in the blanks by selecting the appropriate term from below:
For a process that is non-spontaneous and that favors products at equilibrium, we know that a) ΔrG∘ΔrG∘ _________, b) ΔunivSΔunivS _________, c) ΔsysSΔsysS _________, and d) ΔrH∘ΔrH∘ _________.
Highest occupied molecular orbital
Lowest unoccupied molecular orbital
Label all nodes and regions of highest and lowest electron density for both orbitals.
Relative Intensity
Part VI. consider the multi-step reaction below for compounds A, B, and C.
These compounds were subjected to mass spectrometric analysis and
the following spectra for A, B, and C was obtained.
Draw the structure of B and C and match all three compounds
to the correct spectra.
Relative Intensity
Relative Intensity
20
NaоH
0103
Br
(B)
H2504
→ (c)
(A)
100-
MS-NU-0547
80
40
20
31
10
20
100-
MS2016-05353CM
80
60
100
MS-NJ-09-3
80
60
40
20
45
J.L
80
S1
84
M+
absent
राग
135 137
S2
62
164 166
11
S3
25
50
75
100
125
150
175
m/z
Chapter 17 Solutions
Essential Organic Chemistry, Global Edition
Ch. 17.1 - a. Explain why, when the imidazole ring of...Ch. 17.2 - Prob. 2PCh. 17.3 - Prob. 3PCh. 17.3 - Prob. 4PCh. 17.3 - Prob. 6PCh. 17.4 - Calculate the pI of each of the following amino...Ch. 17.4 - a. Which amino acid has the lowest pI value? b....Ch. 17.5 - What aldehyde is formed when valine is treated...Ch. 17.5 - Prob. 10PCh. 17.5 - Prob. 11P
Ch. 17.5 - Prob. 12PCh. 17.6 - Prob. 13PCh. 17.6 - What amino acid would be formed using the...Ch. 17.6 - What amino acid would be formed when the aldehyde...Ch. 17.7 - Pig liver esterase is an enzyme that catalyzes the...Ch. 17.8 - Prob. 17PCh. 17.8 - Prob. 18PCh. 17.8 - Prob. 19PCh. 17.8 - Prob. 20PCh. 17.10 - Prob. 21PCh. 17.10 - Prob. 22PCh. 17.10 - Why does cyanogen bromide not cleave on the C-side...Ch. 17.10 - Prob. 24PCh. 17.10 - Prob. 26PCh. 17.12 - Prob. 27PCh. 17.13 - a. Which would have the greatest percentage of...Ch. 17 - Draw the predominant form of the following amino...Ch. 17 - What is the pI of serine?Ch. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Which would have a higher percentage of negative...Ch. 17 - Draw the form of aspartate that predominates at...Ch. 17 - Prob. 35PCh. 17 - A professor was preparing a manuscript for...Ch. 17 - a. Why is the pKa of the glutamate side chain...Ch. 17 - Prob. 38PCh. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Three peptides were obtained from a trypsin...Ch. 17 - Prob. 43PCh. 17 - After the polypeptide shown here was treated with...Ch. 17 - The disulfide bridges of a polypeptide were...Ch. 17 - -Amino acids can be prepared by treating an...Ch. 17 - Reaction of a polypeptide with carboxypeptidase A...Ch. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Show how valine can be prepared by a. a Strecker...Ch. 17 - Prob. 51PCh. 17 - Why is proline never found in an -helix?Ch. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 55PCh. 17 - A chemist wanted to test his hypothesis that the...Ch. 17 - A normal polypeptide and a mutant of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A composite material reinforced with aligned fibers, consisting of 20% by volume of silicon carbide (SiC) fibers and 80% by volume of polycarbonate (PC) matrix. The mechanical characteristics of the 2 materials are in the table. The stress of the matrix when the fiber breaks is 45 MPa. Calculate the longitudinal strength? SiC PC Elastic modulus (GPa) Tensile strength (GPa) 400 2,4 3,9 0,065arrow_forwardQuestion 2 What starting materials or reagents are best used to carry out the following reaction? 2Fe, 3Br2 ○ FeCl3 2Fe, 4Br2 O Heat and Br2 Heat and HBr Brarrow_forwardWhat is/are the major product(s) of the following reaction? O AICI -Chts +arrow_forward
- Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. C H H C H :Ö: Click and drag to start drawing a structure.arrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. C H H C. H H H H Click and drag to start drawing a structure. Xarrow_forwardA new brand of lotion is causing skin rush unlike the old brand of the same lotion. With the aid of well labelled diagram describe an experiment that could be done to isolate the pigment that cause the skin rusharrow_forward
- Don't used hand raitingarrow_forwardDon't used hand raitingarrow_forwardRelative Intensity Part VI. consider the multi-step reaction below for compounds A, B, and C. These compounds were subjected to mass spectrometric analysis and the following spectra for A, B, and C was obtained. Draw the structure of B and C and match all three compounds to the correct spectra. Relative Intensity Relative Intensity 100 HS-NJ-0547 80 60 31 20 S1 84 M+ absent 10 30 40 50 60 70 80 90 100 100- MS2016-05353CM 80- 60 40 20 135 137 S2 164 166 0-m 25 50 75 100 125 150 m/z 60 100 MS-NJ-09-43 40 20 20 80 45 S3 25 50 75 100 125 150 175 m/zarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY