
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.6, Problem 14P
What amino acid would be formed using the N-phthalimidomalonic ester synthesis when the following
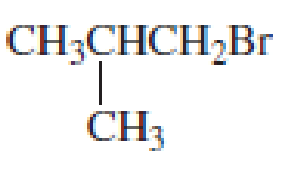
- a. CH3SCH2CH2Br
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In the Thermo Fisher application note about wine analysis (Lesson 3), the following
chromatogram was collected of nine components of wine. If peak 3 has a retention time of
3.15 minutes and a peak width of 0.070 minutes, and peak 4 has a retention time of 3.24
minutes and a peak width of 0.075 minutes, what is the resolution factor between the two
peaks? [Hint: it will help to review Lesson 2 for this question.]
MAU
300
200
T
34
5
100-
1 2
CO
6
7
8
9
0
2.4
2.6
2.8
3.0 3.2 3.4
3.6
3.8 4.0 4.2
4.4
4.6
4.8
5.0
5.2
Minutes
3.22
0.62
1.04
O 1.24
The diagram shows two
metals, A and B, which melt at
1000°C and 1400°C. State the
weight percentage of the
primary constituent (grains of
C) that would be obtained by
solidifying a 20% alloy of B.
1000°C
a+L
L+C
900°С
12
α
a+C
45
1200 C
L+y
140096
C+Y
a+ß
800°C
700°C
C+B
96
92
a+B
0
10
20
30
40
50
60
70 80 90 100
A
% peso B
B
8.
Choose the compound that will produce the spectrum below and assign the signals to the corresponding
protons.
2
4
3
ō (ppm)
OH
4
6 6
СОН
2
1
0
Chapter 17 Solutions
Essential Organic Chemistry, Global Edition
Ch. 17.1 - a. Explain why, when the imidazole ring of...Ch. 17.2 - Prob. 2PCh. 17.3 - Prob. 3PCh. 17.3 - Prob. 4PCh. 17.3 - Prob. 6PCh. 17.4 - Calculate the pI of each of the following amino...Ch. 17.4 - a. Which amino acid has the lowest pI value? b....Ch. 17.5 - What aldehyde is formed when valine is treated...Ch. 17.5 - Prob. 10PCh. 17.5 - Prob. 11P
Ch. 17.5 - Prob. 12PCh. 17.6 - Prob. 13PCh. 17.6 - What amino acid would be formed using the...Ch. 17.6 - What amino acid would be formed when the aldehyde...Ch. 17.7 - Pig liver esterase is an enzyme that catalyzes the...Ch. 17.8 - Prob. 17PCh. 17.8 - Prob. 18PCh. 17.8 - Prob. 19PCh. 17.8 - Prob. 20PCh. 17.10 - Prob. 21PCh. 17.10 - Prob. 22PCh. 17.10 - Why does cyanogen bromide not cleave on the C-side...Ch. 17.10 - Prob. 24PCh. 17.10 - Prob. 26PCh. 17.12 - Prob. 27PCh. 17.13 - a. Which would have the greatest percentage of...Ch. 17 - Draw the predominant form of the following amino...Ch. 17 - What is the pI of serine?Ch. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Which would have a higher percentage of negative...Ch. 17 - Draw the form of aspartate that predominates at...Ch. 17 - Prob. 35PCh. 17 - A professor was preparing a manuscript for...Ch. 17 - a. Why is the pKa of the glutamate side chain...Ch. 17 - Prob. 38PCh. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Three peptides were obtained from a trypsin...Ch. 17 - Prob. 43PCh. 17 - After the polypeptide shown here was treated with...Ch. 17 - The disulfide bridges of a polypeptide were...Ch. 17 - -Amino acids can be prepared by treating an...Ch. 17 - Reaction of a polypeptide with carboxypeptidase A...Ch. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Show how valine can be prepared by a. a Strecker...Ch. 17 - Prob. 51PCh. 17 - Why is proline never found in an -helix?Ch. 17 - Determine the amino acid sequence of a polypeptide...Ch. 17 - Prob. 55PCh. 17 - A chemist wanted to test his hypothesis that the...Ch. 17 - A normal polypeptide and a mutant of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7. Assign all of the protons on the spectrum below. A B 2 C E 2 1 3 6 4 3 2 1 0arrow_forwarde. If (3R,4R)-3,4-dichloro-2,5-dimethylhexane and (3R,4S)-3,4-dichloro-2,5-dimethylhexane are in a solution at the same concentration, would this solution be expected to rotate plane polarized light (that is, be optically active)? Please provide your reasoning for your answer. [If you read this problem carefully, you will not need to draw out the structures to arrive at your answer...]arrow_forward1. How many neighbors does the proton that produces the multiplet below have? 2. 3. اللـ Draw a partial structure from the multiplet below. (The integration of the multiplet is 6) M Using the additivity constants found in appendix G of your lab manual, calculate the approximate chemical shifts of the protons indicated below. (Show your work!!!) B A Br SHarrow_forward
- 1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve this long problem. Thanksarrow_forward4. An 'H-NMR of a compound is acquired. The integration for signal A is 5692 and the integration for signal B is 25614. What is the simplest whole number ratio of protons for signals A and B? (Show your work!!!) 5. Assign the carbons in the NMR below as either carbonyl, aromatic, or alkyl. 200 150 100 50 ō (ppm) 1arrow_forwardSpeaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forward
- If we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forwardWhen natural light falls perpendicularly on a material A, it has a reflectivity of 0.813%. Indicate the value of the refractive index.arrow_forwardIn piezoelectricity and piezoelectric ceramics, one of the following options is false:(A). Piezoelectricity allows an electrical signal to be transformed into a mechanical one.(B). PbZrO3 is a well-known piezoelectric ceramic.(C). Piezoelectricity and ferroelectricity in general have no relationship.(D). One of the applications of piezoelectricity is sonar.arrow_forward
- (30 MARKS) Give the major product(s ) formed including relevant stereochemistry or the complete reaction conditions for the following reactions. More than one step may be required for each reaction arrow, in which case the steps must be numbered 1), 2) etc. (2 marks each box) h) i) h) OH i) HO H3PO4, heat 2 Brarrow_forwardNonearrow_forwardIndicate which option is false(A). Resistivity has a residual component and a thermal component.(B). In some materials resistivity increases with T and in others it decreases.(C). In insulating materials, resistivity is very low.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY