
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 16.8, Problem 29P
Interpretation Introduction
Interpretation:
The
Concept introduction:
The carbonyl group
The general reaction of imine fomrtaion is given as,
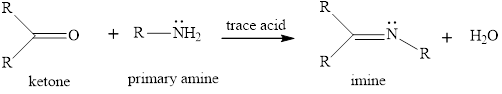
The imine formation is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons
Please sirrr soollveee these parts pleaseeee and thank youuuuu
Please sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseee
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
Ch. 16.1 - Give two names for each of the following:Ch. 16.1 - Prob. 2PCh. 16.1 - Name the following:Ch. 16.2 - Prob. 4PCh. 16.4 - What products are formed when the following...Ch. 16.4 - We saw on the previous page that...Ch. 16.4 - a. How many stereoisomers are obtained from the...Ch. 16.4 - Prob. 9PCh. 16.4 - Write the mechanism for the reaction of acetyl...Ch. 16.4 - Prob. 11P
Ch. 16.4 - Show how the following compounds can be...Ch. 16.4 - Prob. 13PCh. 16.4 - Prob. 14PCh. 16.4 - In the mechanism for cyanohydrin formation, why is...Ch. 16.4 - Prob. 16PCh. 16.4 - Prob. 17PCh. 16.4 - Show two ways to convert an alkyl halide into a...Ch. 16.5 - Prob. 20PCh. 16.5 - Prob. 21PCh. 16.5 - Prob. 22PCh. 16.5 - Prob. 23PCh. 16.6 - Prob. 24PCh. 16.7 - What reducing agents should be used to obtain the...Ch. 16.7 - Prob. 26PCh. 16.8 - Prob. 27PCh. 16.8 - Prob. 28PCh. 16.8 - Prob. 29PCh. 16.8 - The pKa of protonated acetone is about 7.5. and...Ch. 16.8 - Prob. 31PCh. 16.8 - Prob. 32PCh. 16.8 - Prob. 33PCh. 16.8 - Excess ammonia must be used when a primary amine...Ch. 16.8 - The compounds commonly known as amino acids are...Ch. 16.9 - Hydration of an aldehyde is also catalyzed by...Ch. 16.9 - Which ketone forms the most hydrate in an aqueous...Ch. 16.9 - When trichloroacetaldehyde is dissolved in water,...Ch. 16.9 - Which of the following are a. hermiacetals? b....Ch. 16.9 - Prob. 40PCh. 16.9 - Explain why an acetal can be isolated but most...Ch. 16.10 - Prob. 42PCh. 16.10 - Prob. 43PCh. 16.10 - What products would be formed from the proceedings...Ch. 16.10 - a. In a six-step synthesis, what is the yield of...Ch. 16.10 - Show how each of the following compounds could be...Ch. 16.12 - Prob. 47PCh. 16.13 - Prob. 49PCh. 16.14 - Prob. 50PCh. 16.15 - Prob. 51PCh. 16.16 - Prob. 52PCh. 16 - Draw the structure for each of the following: a....Ch. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - a. Show the reagents required to form the primary...Ch. 16 - Prob. 58PCh. 16 - Prob. 59PCh. 16 - Using cyclohexanone as the starting material,...Ch. 16 - Propose a mechanism for each of the following...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Fill in the boxes:Ch. 16 - Prob. 64PCh. 16 - Identify A through O:Ch. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - How many signals would the product of the...Ch. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Prob. 73PCh. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - Prob. 78PCh. 16 - Draw structure for A-D for each of the following:Ch. 16 - Prob. 80PCh. 16 - a. Propose a mechanism for the following reaction:...Ch. 16 - Prob. 82PCh. 16 - A compound gives the following IR spectrum. Upon...Ch. 16 - How can be following compounds be prepared from...Ch. 16 - Prob. 85PCh. 16 - Prob. 86PCh. 16 - Prob. 87PCh. 16 - In the presence of an acid catalyst, acetaldehyde...Ch. 16 - Prob. 89PCh. 16 - Prob. 90PCh. 16 - Prob. 91PCh. 16 - A compound reacts with methylmagnesium bromide...Ch. 16 - Show how each of the following compounds can be...Ch. 16 - Prob. 94PCh. 16 - The pKa values of the carboxylic acid groups of...Ch. 16 - The Baylis-Hillman reaction is a DABCO...Ch. 16 - Prob. 97PCh. 16 - Prob. 98P
Knowledge Booster
Similar questions
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
- Select all molecules which are chiral. Brarrow_forwardUse the reaction coordinate diagram to answer the below questions. Type your answers into the answer box for each question. (Watch your spelling) Energy A B C D Reaction coordinate E A) Is the reaction step going from D to F endothermic or exothermic? A F G B) Does point D represent a reactant, product, intermediate or transition state? A/ C) Which step (step 1 or step 2) is the rate determining step? Aarrow_forward1. Using radii from Resource section 1 (p.901) and Born-Lande equation, calculate the lattice energy for PbS, which crystallizes in the NaCl structure. Then, use the Born-Haber cycle to obtain the value of lattice energy for PbS. You will need the following data following data: AH Pb(g) = 196 kJ/mol; AHƒ PbS = −98 kJ/mol; electron affinities for S(g)→S¯(g) is -201 kJ/mol; S¯(g) (g) is 640kJ/mol. Ionization energies for Pb are listed in Resource section 2, p.903. Remember that enthalpies of formation are calculated beginning with the elements in their standard states (S8 for sulfur). The formation of S2, AHF: S2 (g) = 535 kJ/mol. Compare the two values, and explain the difference. (8 points)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning